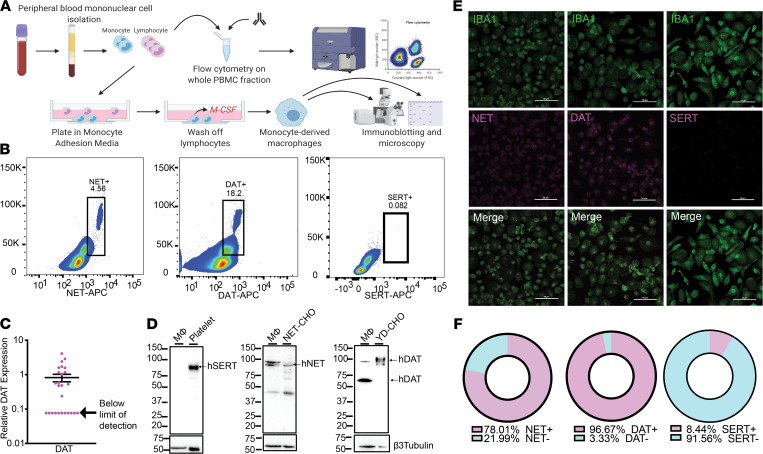
Figure 1. Human monocytes and monocyte-derived macrophages express NET and DAT, but not SERT.
(A) Schematic depicting the isolation of PBMCs from human whole blood via density-dependent centrifugation with Ficoll. A fraction of the isolated PBMCs was used for flow cytometric analysis, and the remaining cells were plated in monocyte-adhesion media with autologous serum and allowed to differentiate into monocyte-derived macrophages. (B) Density plots of flow cytometry data on acutely isolated human PBMCs show that approximately 18.2% of circulating monocytes are DAT+ and approximately 4.56% of circulating monocytes are NET+ (scatter plots representative of 3 independent experiments). SERT was not detected on monocytes. (C) qPCR on cultured human monocyte-derived macrophages indicates that the mRNA for DAT protein is expressed in these cells (n = 26). (D–F) Cultured human monocyte-derived macrophages were either prepared for Western blot analysis (D), or immunocytochemistry (E and F). Representative Western blots from lysates of cultured human monocyte-derived macrophages probed for SERT, NET, or DAT. Human monocyte-derived macrophages did not express SERT (positive control: human platelets) but did express both NET (positive control: NET-expressing CHO cells) and DAT (positive control: YFP-DAT-expressing CHO cells) (n = 3 independent experiments). (E) Representative confocal images of human monocyte-derived macrophages immunostained for IBA1 and either NET, DAT, or SERT. IBA+ cells (macrophages) were positive for NET and DAT, but not SERT (n = 3 independent experiments). (F) Threshold-based quantification of NET+ (top), DAT+ (middle), and SERT+ (bottom) IBA1+ macrophages based on images in E indicating that 78% of macrophages were NET+, nearly 97% were DAT+, and only 8% were SERT+.