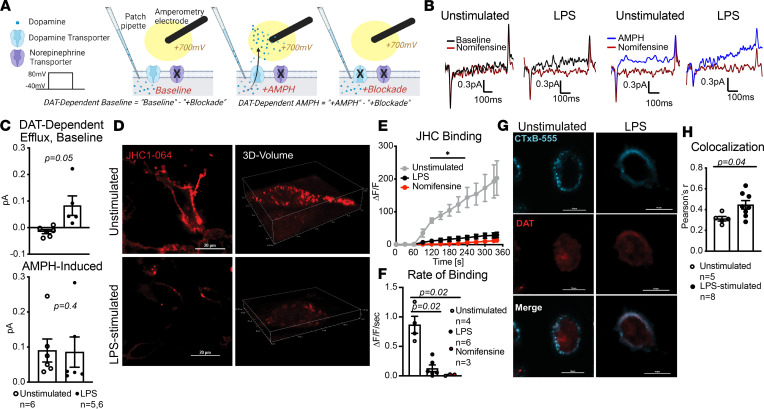
Figure 7. LPS-stimulation increased DAT-mediated dopamine efflux and decreased DAT–JHC1-064 binding.
(A) Schematic representation of experimental design using simultaneous whole-cell patch-clamp and amperometry technique to measure DAT-mediated dopamine efflux. (B) Representative amperometric traces from unstimulated and LPS-stimulated human macrophages. (C) Bar graphs show basal or amphetamine-induced DAT-mediated dopamine efflux. While unstimulated macrophages did not exhibit measurable DAT-mediated dopamine efflux at baseline, LPS-stimulation significantly increased basal DAT-mediated dopamine efflux (top, P = 0.05, Welch’s 2-tailed t test) but did not further increase or decrease the amphetamine-induced dopamine efflux (bottom, P = 0.9, Mann Whitney U test). Data are from n = 5–6 experiments/group. (D) Representative 40× images of JHC1-064 binding to unstimulated and LPS-stimulated macrophages. (E) Quantification of fluorescence signal showed that LPS significantly decreased the magnitude of JHC1-064–DAT binding to levels similar to those seen with DAT blockade (nomifensine-treated, *P < 0.05, 2-way ANOVA with Tukey’s post hoc test for multiple comparisons). (F) The average rate of JHC1-064–DAT binding (average slope from E) was similarly decreased by LPS-stimulation (P = 0.02) and by presence of DAT-specific antagonist, nomifensine (P = 0.02). Data in D–F are from n = 3–6 independent experiments/group, and statistical analysis was performed by Brown-Forsythe ANOVA with Tukey’s post hoc test for multiple comparisons. (G) Representative images of unstimulated and LPS-stimulated macrophages labeled with CTxB-555 and DAT. (H) Quantifying colocalization between GM-1 and DAT using Pearson’s correlation coefficient showed LPS-stimulated macrophages has significantly increased DAT-CTxB colocalization compared with unstimulated macrophages (P = 0.04, unpaired 2-tailed t test). Images and data in G and H are from n = 5 and 8 experiments for unstimulated and LPS-stimulated groups, respectively.