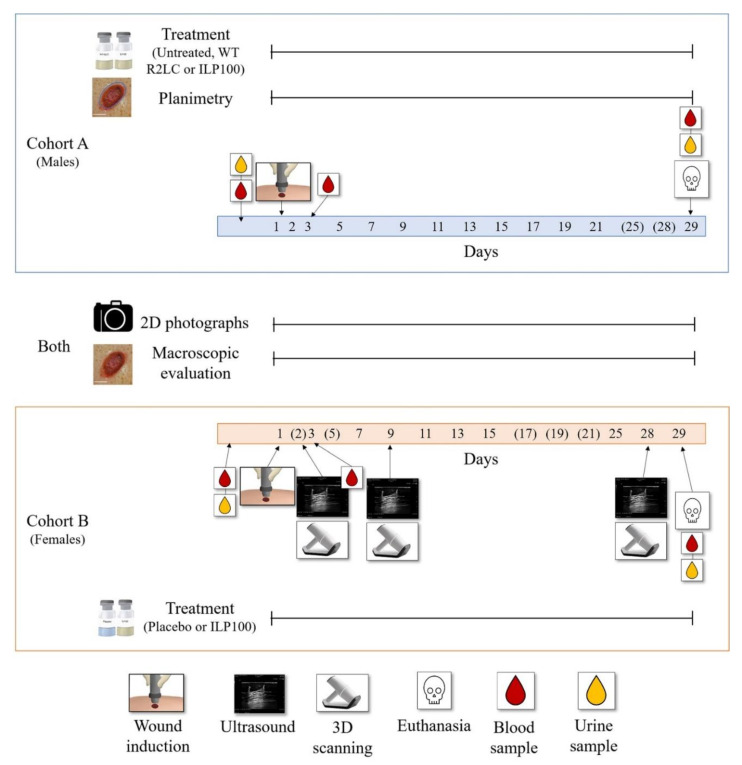
Figure 1.
Schematic illustration of the protocol design. The study consists of two cohorts: one in males (Cohort A, blue box) and one in females (Cohort B, orange box). In the illustration, the numbers in the filled blue (Cohort A) and orange (Cohort B) boxes indicate the days where 2D photographs, macroscopic evaluation, treatment, and planimetry (only Cohort A) were performed. No 2D photographs, macroscopic evaluation, or planimetry were performed on the days in parenthesis. Time points for wound induction, collection of blood (for hematology, clinical chemistry, plasma levels of CXCL12 and SppIP, and for CFU counts of ILP100), collection of urine samples, and for additional imaging (only Cohort B) with ultrasound and 3D scanning are indicated with arrowed symbols.