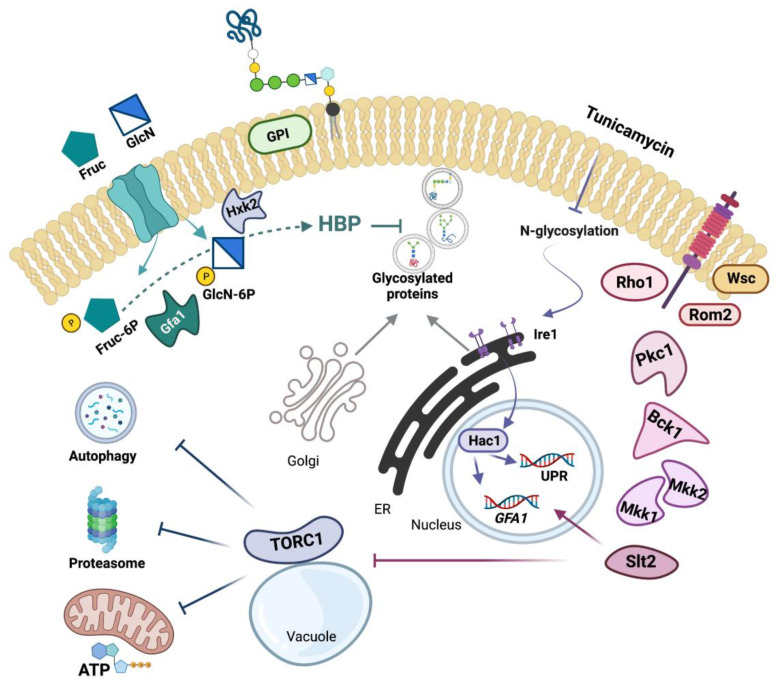
Figure 9.
Schematic representation of the ER-stress signaling network and its interaction with Slt2 and the hexosamine pathway. The CWI signaling pathway from the anchored Wsc sensors to the final MAPK Slt2 (see [3,5,6,7,8,9,10] as representative reviews) and the metabolic steps from fructose (Fruc) or glucosamine (GlcN) to glucosamine-6-phosphate (GlcN-6P) catalyzed by Gfa1 [22] and Hxk2 [49,50], respectively, are shown. Gfa1 catalyzes the first committed and rate-limiting step of the HBP, which provides UDP-N-acetylglucosamine for, among others, glycosylation and GPI-anchoring of proteins [20,21]. Tunicamycin exposure induces ER stress by inhibiting the N-glycosylation of proteins [42], which triggers a protective response, the UPR, a transcription program mediated by Ire1 and Hac1, that upregulates the transcription of hundreds of genes [13,14,15,16], among them GFA1 [24]. Expression of GFA1 also depends on Slt2 [26], which link the CWI and the UPR in providing increased flux through the HBP under ER-stress conditions, a response that reduces the abundance of ER-client proteins. ER-stress also inhibits TORC1 [66,67], a stress and growth controller [63], which downregulates protein homeostasis mediated by autophagy [64,78] and proteasome [68] activities and the bioenergetics response [84,86], which provides the energy required for protein folding and clearance of protein aggregates under ER stress [85]. Remarkably, Slt2 is required to both GFA1 expression and TORC1 inhibition in response to ER-stress, which accounts for the strong growth defect of cells devoted of Slt2 in media containing ER-stress-inducers, a phenotype that can be relieved by GFA1 overexpresion or GlcN supplementation. Arrows and bars denote positive and negative interactions, respectively. For additional details, see the text.