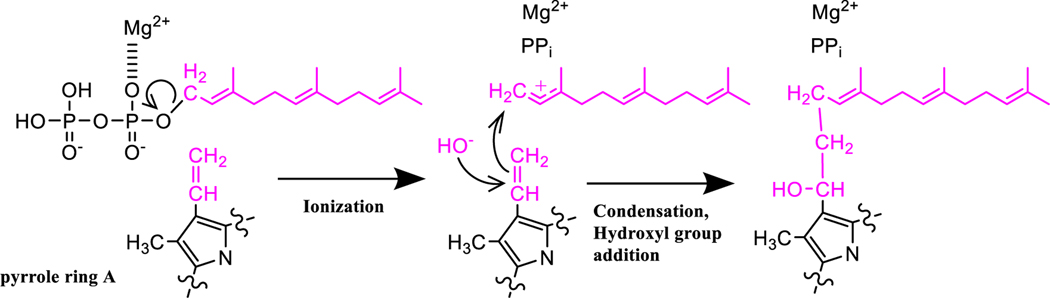
Figure 2. Proposed reaction mechanism for the conversion of heme b to heme o.
HOS catalyzes the transfer of a farnesyl moiety (in pink) from farnesyl diphosphate to the vinyl group of heme b (in pink) at pyrrole ring A. For simplicity, only pyrrole ring A of the heme is shown. Ionization of farnesyl diphosphate yields a farnesyl cation intermediate stabilized by delocalization across C′1-C′3. Interactions with Mg2+ ions (only one shown here) make the pyrophosphate a better leaving group and allow ionization to proceed. The vinyl group then attacks the carbocation intermediate to form a new C-C bond (condensation), and a hydroxyl group is added to C1 to produce heme o. A basic residue in the active site could promote the formation of a hydroxyl group prior to the attack of C1, or water could attack C1 and subsequently be deprotonated. The timing of the main three steps (ionization, condensation, and hydroxyl group addition) has not been established for HOS. For example, ionization and condensation may be concerted. Additionally, condensation may precede addition of the hydroxyl group, or these two steps may occur simultaneously. For heme variants oT and oP1, the hydroxyethylfarnesyl group is replaced by an ethylprenyl group (C15 or C20, respectively), indicating that the catalytic activity of HOS in certain archaea differs slightly from the canonical HOS mechanism shown here (Lübben and Morand 1994).