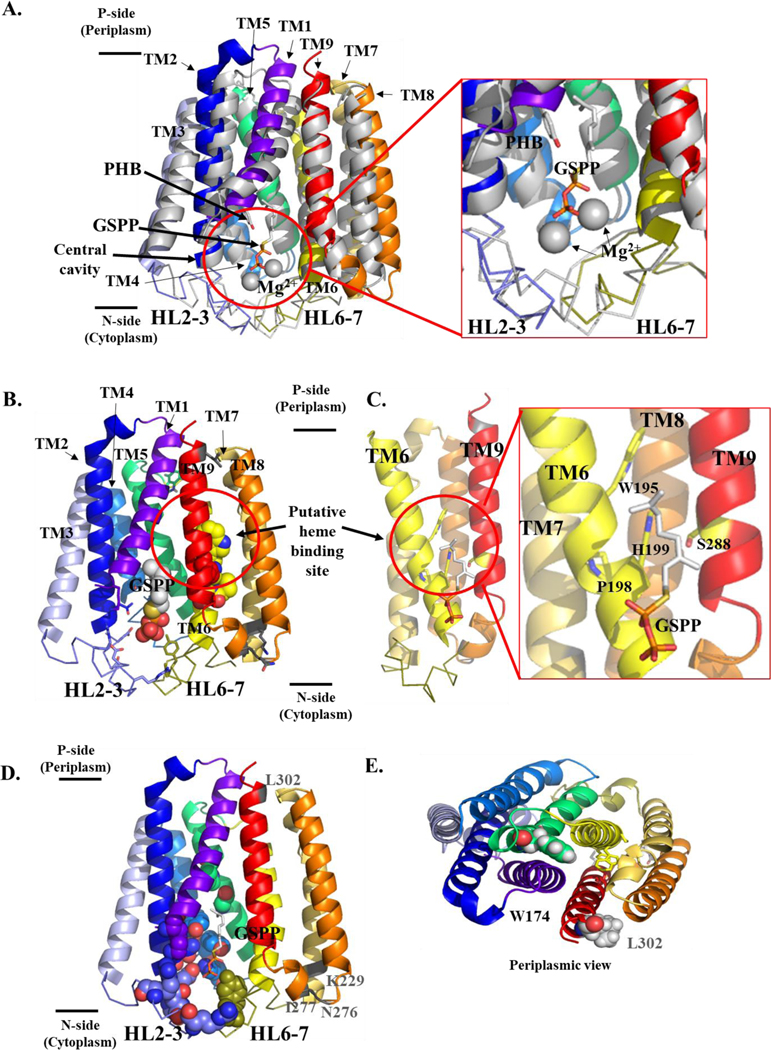
Figure 3. Structural model of B. subtilis HOS showing the cytoplasmic central cavity and predicted heme binding site.
A. Superimposition of substrate-bound ApUbiA, shown in gray, (PDB 4OD5) (Cheng and Li 2014) and the BsHOS model (colored according to secondary structure). The prenyl acceptor, PHB, (gray and red) and the prenyl donor analog, GSPP (gray, red, and orange) are shown as sticks. Mg2+ ions are shown as gray spheres. BsHOS model coloring: TM1 purple-blue, TM2 blue, HL2–3 slate (ribbon), TM3 light blue, TM4 marine, TM5 lime green, TM6 yellow, HL6–7 deep olive (ribbon), TM7 yellow-orange, TM8 orange, TM9 red. The N-terminus (aa 1–28) of BsHOS is not shown. B. BsHOS model viewed from the plane of the membrane. Placement of GSPP (shown as spheres) was based on the superimposition of the BsHOS model with the ApUbiA structure as shown in panel A. The critical, central cavity-facing residues are shown as sticks (colors corresponding to secondary structure). The extramembrane residues (charged in EcHOS, varying levels of conservation) are shown as gray sticks. The putative heme binding site between TM6 (yellow) and TM9 (red) is circled. Putative heme binding site residues are shown as spheres (yellow and red). C. The model is rotated 90° to show the putative heme binding site adjacent to the central cavity. For clarity, TM1–5 have been removed. Critical residues in the putative heme binding site are shown as yellow sticks, and GSPP is shown as sticks. D. View of the BsHOS model from the TM plane, highlighting critical residues that face the central cavity as well as charged, extramembrane residues that do not face the central cavity but are critical in EcHOS. Key central cavity residues are shown as spheres. The backbone positions of four “charged” extramembrane key residues are shown in gray and labeled in gray. The substrate analog GSPP is shown as sticks. E. Periplasmic view of W174, a critical residue that does not face the central cavity. W174 is shown in green spheres. L302 (analogous to the critical charged, extramembrane residue D282 in EcHOS) is shown as gray spheres. GSPP – geranyl thiolopyrophosphate; PHB – p-hydroxybenzoate; HL – helix-loop; TM – transmembrane helix.