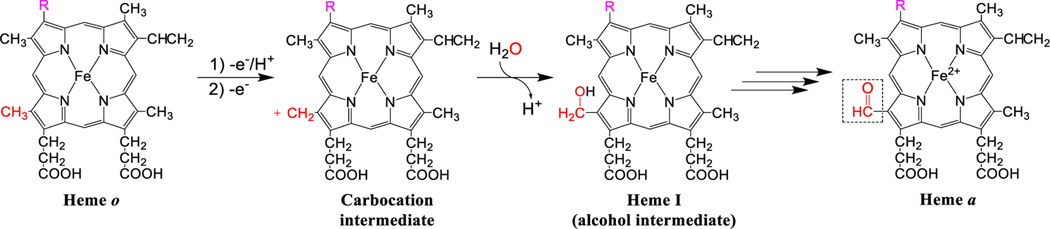
Figure 4. Overview of the proposed reaction mechanism for the conversion of heme o to heme a.
The C-8 methyl group (shown in red) loses a proton and two electrons, generating a carbocation. An oxygen atom from water (shown in red) traps this carbocation, yielding heme I, an alcohol intermediate. A second oxidation step ultimately converts the alcohol into an aldehyde, generating heme a.