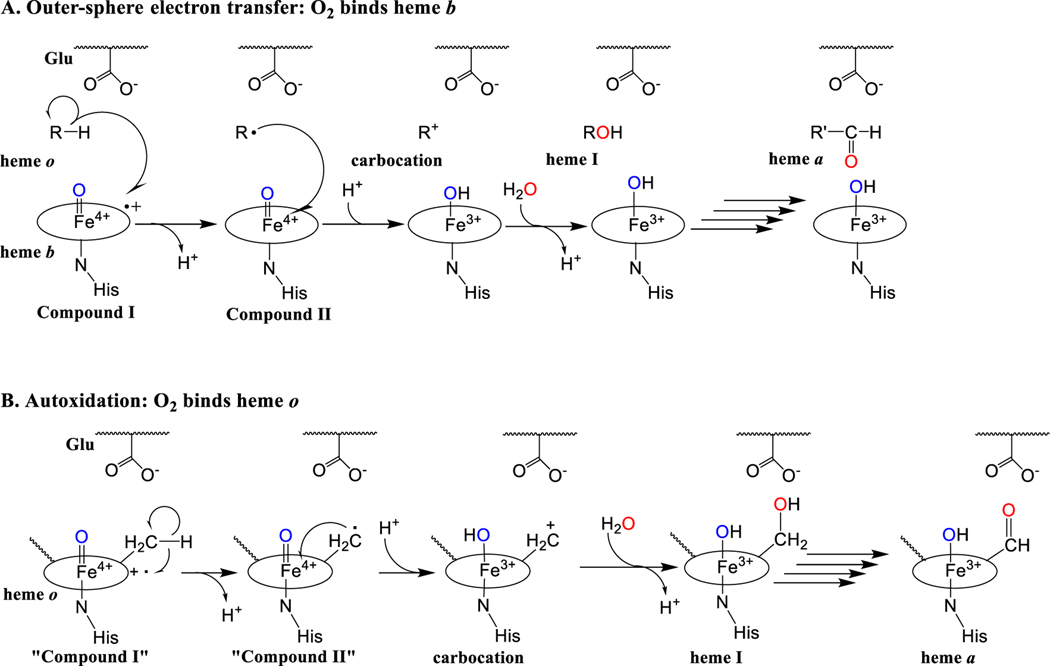
Figure 5. Possible mechanisms of oxygen activation by HAS.
In HAS, O2 activation may occur at heme b (A) or heme o (B). Either proposed mechanism would likely require the displacement of a histidine ligand to allow O2 to bind. In both mechanisms, substrate oxidation is shown as two successive one-electron transfer steps instead of hydrogen atom abstraction because the substrate (the C8 methyl group of heme o) is not positioned appropriately relative to the ferryl group in compound I to allow hydrogen atom abstraction. A. Outer-sphere electron transfer. O2 activation leads to the formation of a high-valent iron-oxo species (compound I, far left) that removes an electron from the C8 methyl group of heme o (shown as R-H). This leads to the formation of a radical intermediate and compound II. Compound II then removes another electron from the substrate radical, forming a carbocation intermediate. Water traps the carbocation intermediate to form heme I. This process can then be repeated with heme I as the substrate to form a geminal diol (not shown), which readily dehydrates to form the aldehyde in heme a, the final product. A conserved glutamate positioned near the heme o binding site is proposed to stabilize the carbocation intermediate. B. Autoxidation mechanism. Heme o activates O2 to form a compound I-like species. “Compound I” of heme o then oxidizes its own C8 methyl group using the general mechanism described in mechanism A.