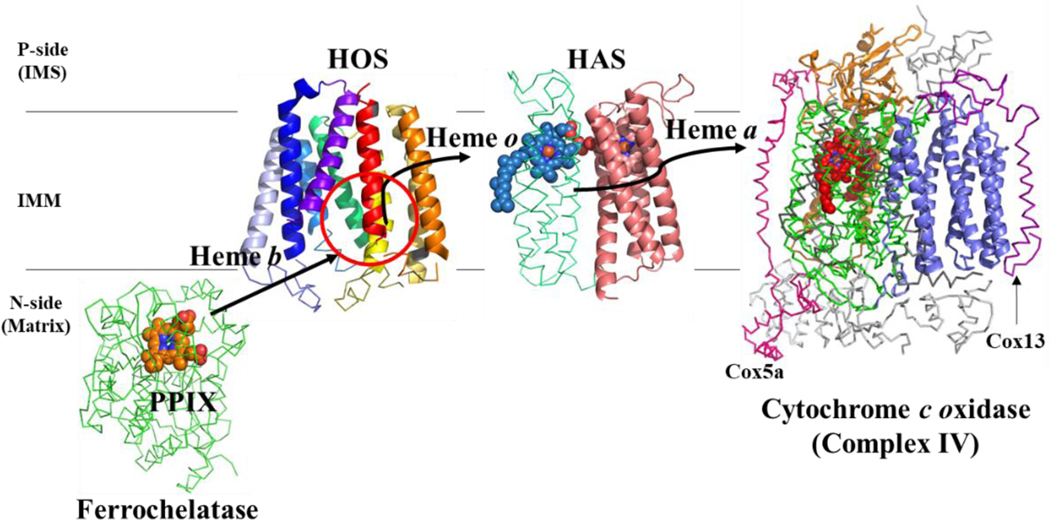
Figure 7. Proposed heme trafficking route in the eukaryotic heme a biosynthetic pathway.
Heme must be transferred from ferrochelatase to HOS, from HOS to HAS, and finally from HAS to cytochrome c oxidase. Note that these transfer steps could involve an unknown heme chaperone (see text). Proteins shown: Membrane-associated (eukaryotic) ferrochelatase (green ribbon) is shown in complex with protoporphyrin IX (PPIX) (orange spheres). The porphyrin plane is roughly parallel to the membrane lipids, and pyrrole rings A and D are closest to the membrane. Note that while mammalian ferrochelatase can dimerize, only one monomer is shown here (Burden et al. 1999; Wu et al. 2001; Medlock et al. 2007). (Ferrochelatase structure (human): PDB E343K) (Medlock et al. 2007). HOS model (this study) is shown with the proposed heme binding site circled in red (BsHOS). HAS model (Niwa et al. 2018) showing heme o (blue spheres) bound in the N-terminal heme binding site on the P-side (periplasmic or IMS side) of the membrane. This model is based on the crystal structure of BsHAS, which has an empty N-terminal heme binding site (model PDB file: pnas.1813346115.sd01). Cytochrome c oxidase is depicted as in Figure 1A, except that hemes a and a3 are shown as red spheres. Subunit I (Cox1) is shown in green ribbon; the other core subunits are shown as orange and slate cartoons. The nuclear-encoded subunits are shown as gray ribbons, except for Cox5a (hot pink) and Cox13 (purple). IMS – intermembrane space; IMM – inner mitochondrial membrane; P-side – positive side; N-side – negative side.