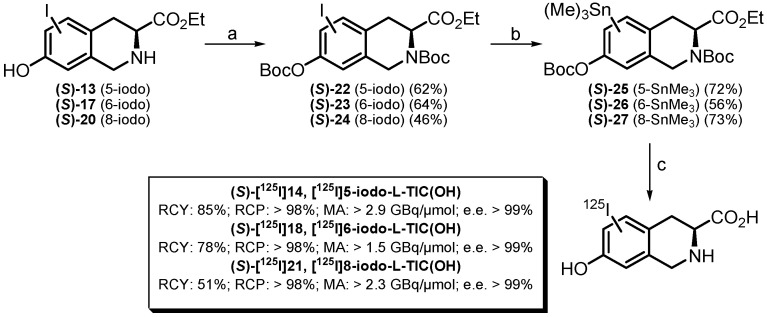
Scheme 4.
Preparation of [125I]iodo-L-TIC(OH) compounds ((S)-[125I]14, (S)-[125I]18 and (S)-[125I]21). Reagents and conditions: (a) Boc2O, Et3N, DMAP, CH2Cl2, r.t., 16–20 h; (b) Sn2Me6, Pd(PPh3)4, 1,4-dioxane, reflux, 1.5 h for (S)-25 and (S)-26 or (i) 1.3 M iPrMgCl.LiCl, THF, −40 °C, 20 min; (ii) Me3SnCl, THF, −40 °C, 3 h then r.t. for (S)-27; (c) (i) [125I]NaI, CAT, EtOH, 1M PBS buffer, r.t., 5 min; (ii) aq. NaOH 10 M, 0 °C then r.t., 1 h; (iii) TFA, 0 °C then 60 °C, 30 min.