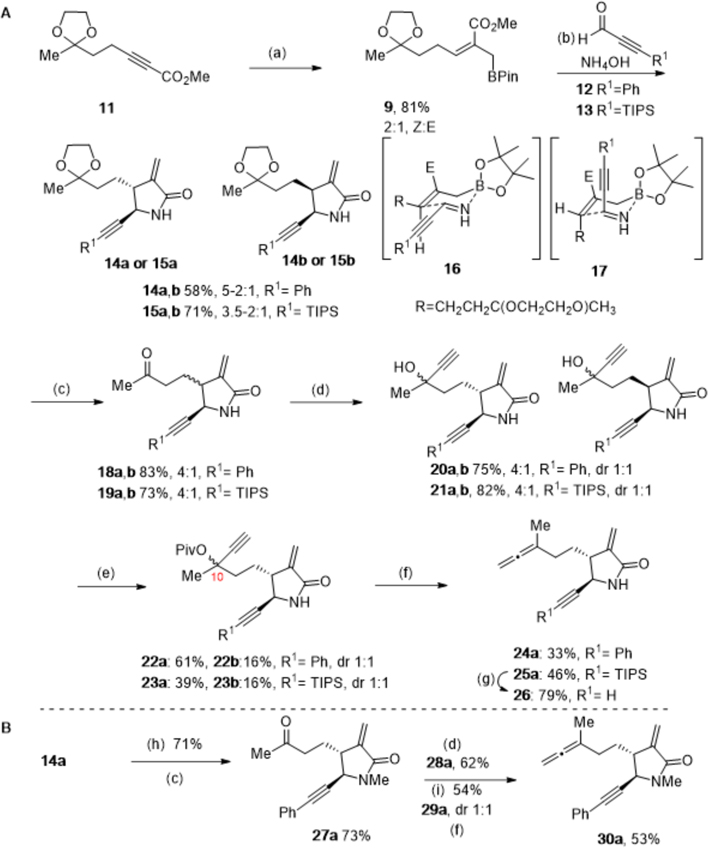
Scheme 2. Synthesis of α-Methylene-γ-lactam Tethered Allene-ynes.
Reagents and conditions: (a) CuI, MeLi in Et2O, THF, −30 °C, 30 min; then toluene, HMPA, DIBALH in hexanes, −30 °C, 2 h; then 11, −20 °C, 5 h; then 2-(chloromethyl)-4,4,5,5-tetramethyl-1,3-dioxaborolane (PinBCH2Cl), −20 °C to rt, 16 h, 76% as a 3:1; Z:E mixture along with methyl (Z,E)-5-(2-methyl-1,3-dioxolan-2-yl)pent-2-enoate (S4), 10%; (b) 12 or 13, ammonium hydroxide, ethanol, rt, 16 h; (c) PPTS, acetone:water (15:1), reflux, 16 h; (d) ethynyl magnesium bromide, THF, 0 °C, 3 h; (e) scandium triflate, pivalic anhydride, CH3CN, rt, 16 h; (f) triphenylphosphine copper hydride hexamer (Stryker’s reagent), toluene, −10 °C, 2 h; (g) tetra-n-butyl ammonium fluoride, THF, 0 °C, 45 min; (h) sodium hydride, iodomethane, DMF, 0 °C to rt, 15 min; (i) acetic anhydride, Et3N, DMAP, CH2Cl2, 0 °C to rt, 3 h.