Table 1.
Optimization of Allene Formation with Stryker’s Reagent ([CuHPPh3]6).
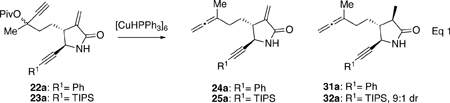
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | SM | Equiv Stryker’s | Temp (°C) | Time (h) | Total yield (%) | (24a/25a):(31a/32a):SM | Scale (mg) |
|
| |||||||
| 1 | 23a | 1.8 | 0 | 1.5 | 64 | 100:trace:0 | 110a |
|
| |||||||
| 2 | 23a | 1 | 0 | 1 | 73 | 60:40:0 | 110 |
|
| |||||||
| 3 | 23a | 0.8 | −10 | 2 | 66 | 63:25:12 | 110 |
|
| |||||||
| 4 | 23a | 0.9 | −20 | 2 | 58 | 80:20:0 | 110 |
|
| |||||||
| 5 | 23a | 0.9 | −10 | 2 | 78 | 77:23:0 | 220 |
|
| |||||||
| 6 | 23a | 0.9 | −10 | 2 | 49 | 72:19:9 | 440 |
|
| |||||||
| 7 | 22a | 0.9 | −10 | 2 | 70 | 70:16:0b | 110 |
|
| |||||||
| 8 | 22a | 0.9 | −10 | 1.5 | 56 | 90:10:0 | 122 |
SM = starting material
Reaction performed with a previously-opened aged container of Stryker’s reagent; all other reactions were performed with the newly opened container
14% of the total yield was a product resulting from reduction of the alkyne.
