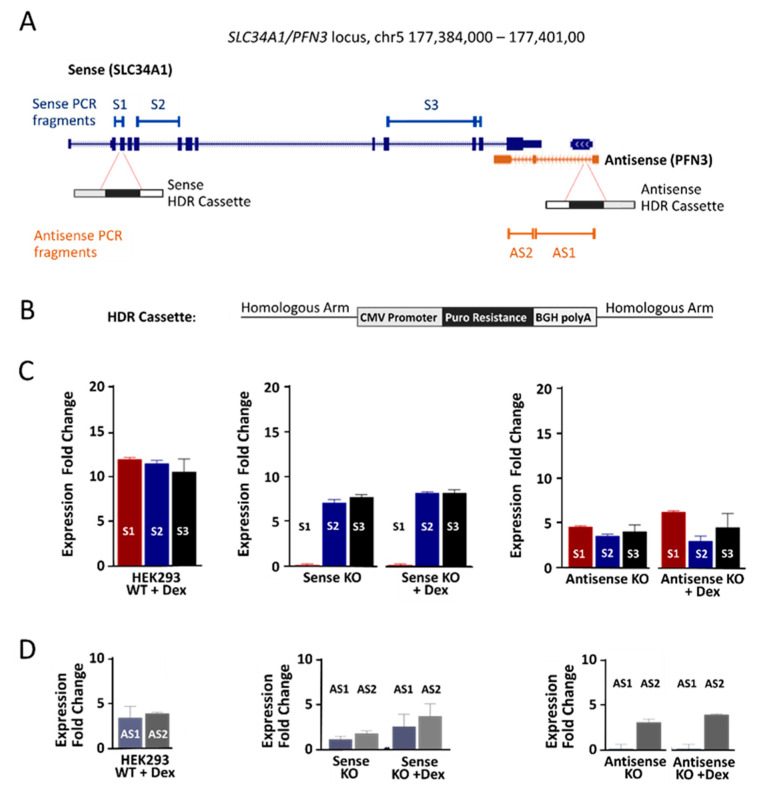
Figure 5.
Constitutive low-level activation of sense and antisense expression in HEK293 cells. (A) Snapshot of the SLC34A1/PFN3 locus with the insertion sites for the HDR (homology-directed repair) cassettes and primer sites S1–S3 as well as AS1 and AS2. (B) Schematic representation of the HDR cassette containing a CMV promoter, the puromycin resistance, the BGH polyadenylation signal and the two gene-specific flanking regions (homologous arm). The cassette was meant to shut down transcription, but insertion produced low levels of read-through transcripts driven by the CMV promoter. (C) Sense transcript expression in cells with the knock-in construct in sense orientation (middle) and antisense orientation (right). Wildtype HEK293 cells stimulated with dexamethasone (left) were used as positive controls; all expression levels were referred to unstimulated HEK293 wildtype cells. The bars represent specific primer pairs and are color coded, S1 red, S2 blue and S3 black. Of note, primer pair 1 is upstream of the cassette insertion site and does not amplify the read-through transcript. (D) Antisense transcript expression in CRISPR edited HEK293 cell clones, the left panel shows unedited HEK293 cells exposed to dexamethasone as a control. Monoallelic insertion of the cassette placed in sense (middle) and antisense (right) orientation. The primer pair AS1 (blueish) flanks the cassette and only generates a PCR product from clones without an insertion. Data are the mean from three independent biological and three technical replicates. Expression levels are normalized to GAPDH, and the fold change (2−ΔΔCT) was calculated in comparison to the non-treated HEK293 cells.