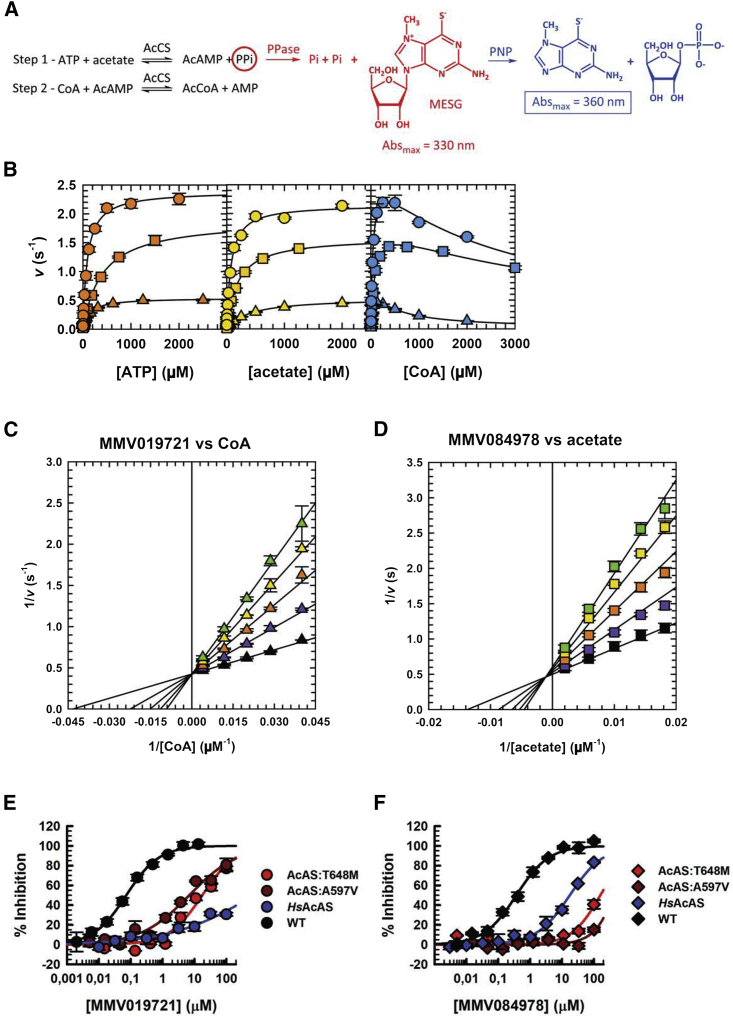
Figure 4.
PfAcAS steady-state kinetics and inhibition by MMV019721 and MMV084978
(A) PfAcAS reaction mechanism and EnzChek assay readout.
(B) Steady-state kinetics of PfAcAS WT (circles), A597V (triangles), and T648M (squares). Saturation curves for ATP (orange), acetate (yellow), and CoA (blue). Error bars indicate the SD, n = 3. Lines are the best fit to Equations 1 (ATP and acetate) and 2 (CoA) in the STAR Methods.
(C) Double-reciprocal plot illustrating the linear competitive inhibition pattern obtained when varying the concentration of MMV019721 at fixed variable concentrations of CoA and saturating concentrations of ATP and acetate. Points are data obtained with 0 (black), 20 (purple), 40 (orange), 60 (yellow), and 80 nM (green triangles) of MMV019721. The error bars indicate the SD, n = 3. Lines are the best fit of the entire dataset to Equation 4.
(D) Double-reciprocal plot illustrating the linear mixed inhibition pattern obtained when varying the concentration of MMV084978 at fixed variable concentrations of acetate and saturating concentrations of ATP and CoA. Points are data obtained with 0 (black), 20 (purple), 40 (orange), 60 (yellow), and 80 nM (green squares) of MMV084978. The error bars indicate the SD, n = 3. Lines are the best fit of the entire dataset to Equation 5.
Saturation curves for MMV019721 (E) and MMV084978 (F) against PfAcAS WT (black symbols), A597V (dark red symbols), T648M (red symbols), and HsAcAS (blue symbols). Error bars indicate the SD, n = 3. Lines are the best fit to Equation 3 and a linear fit for MMV084978 against PfAcAS A597V (dark red line).
See also Figures S4 and S5 and Tables S5 and S6.