Abstract
The present study was the first to evaluate the phytochemical composition, antioxidant, antimicrobial, antibiofilm, and anti-quorum sensing potential of Allium subhirsutum L. (hairy garlic) aqueous extract through in vitro and in silico studies. The phytochemical profile revealed the presence of saponins, terpenes, flavonols/flavonones, flavonoids, and fatty acids, particularly with flavonoids (231 ± 0.022 mg QE/g extract), tannins (159 ± 0.006 mg TAE/g extract), and phenols (4 ± 0.004 mg GAE/g extract). Gas chromatography–mass spectrometry (GC–MS) analysis identified 15 bioactive compounds, such as 5-hydroxymethylfurfural (37.04%), methyl methanethiolsulfonate (21.33%), furfural (7.64%), beta-D-glucopyranose, 1,6-anhydro- (6.17%), 1,6-anhydro-beta-D-glucofuranose (3.6%), trisulfide, di-2-propenyl (2.70%), and diallyl disulfide (1.93%). The extract was found to be non-toxic with 50% cytotoxic concentration higher than 30,000 µg/mL. The investigation of the antioxidant activity via DPPH (2, 2-diphenyl-1-picrylhydrazyl) and FRAP (IC50 = 1 μg/mL), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); IC50 = 0.698 ± 0.107 μg/mL), and β-carotene (IC50 = 0.811 ± 0.036 mg/mL) was assessed. Nevertheless, good antimicrobial potential against a diverse panel of microorganisms with bacteriostatic and fungistatic effect was observed. Quorum sensing inhibition effects were also assessed, and the data showed the ability of the extract to inhibit the production of violacein by the mutant C. violaceum strain in concentration-dependent manner. Similarly, the biofilm formation by all tested strains was inhibited at low concentrations. In silico pharmacokinetic and toxicological prediction indicated that, out of the sixteen identified compounds, fourteen showed promising drug ability and could be used as lead compounds for further development and drug design. Hence, these findings support the popular use of hairy garlic as a source of bioactive compounds with potential application for human health.
Keywords: Allium subhirsutum L., phytochemistry, antioxidant, antimicrobial, antibiofilm, anti-quorum sensing, pharmacokinetics, toxicological prediction
1. Introduction
In modern medicine, many bacteria and their associated infections are considered the major challenge in public health worldwide, causing annually 35,000 deaths in the United States and about 33,000 deaths in European Union [1,2]. Furthermore, the treatments with synthetic drugs are often associated with higher side effects and cannot be tolerated by some people at high doses. Contrary, the exploration of herbs and plant-based products remains important and one of the most applied pharmacological alternatives for the prevention and the treatment of several pathologies as well as a large number of illnesses due to their safety, affordability, and availability [3,4]. Their richness in bioactive molecules such as polyphenols and biochemical components of phytomedicines (alkaloids, flavonoids, phenolics, carotenoids, polysaccharides, lactones, and tannins, …) gives them potent therapeutic benefits, allowing them to be a potential escort to the development of new drug candidates [5,6,7,8,9,10,11,12]. They have a powerful action in reducing the threat of numerous chronic diseases caused by free radicals, destroying the immune system, and translating into serious oxidative stress and oxygen (O2) detoxification [12,13].
Besides that, the spread of infection disease, as well as the emergence of multidrug-resistant bacteria and fungi, is increased recently due to the failure of chemotherapy and the indiscriminate or frequently uses of antibiotics, and their inhibition by alternative agents become an urgent need. Therefore, attention is now being shifted towards natural active components isolated from herbs along with phytomedicine which shares high antioxidant and antimicrobial effects with low cost and high efficiency. As a result, they have been used to avoid various curable infection diseases as the WHO has pointed out that ‘‘no action today means no cure tomorrow”.
In this respect, Allium subhirsutum L. is a perennial plant that belongs to the garlic family. Recent literature conducted by our team reported on the ethno-pharmacological use of bulbs for therapeutic claims, including antioxidant, anti-inflammatory, and anticancer claims, as well as those relating to the inhibition of tumor angiogenesis in a murine model of skeletal metastases [14,15]. Based on an evaluation of our previous study, the phytochemical analysis of this plant has only been assessed by HR-LCM for methanolic extract; the results from this indicate that it contains various phytoconstituents, particularly polyphenols and flavonoids, along with several other bioactive compounds [15,16,17,18,19,20,21]. Whole plant is used in the popular medicine in Sardinia (Italy) due to its anti-hemorrhoidal, blood pressure regulation, and purifying action [22]. In addition, hairy garlic buds and leaves are cut into small pieces and eaten raw or used as flavoring agent in cooked dishes and salads [23]. It is also a good ingredient in the traditional Turkish flat bread called yufka [24]. Few studies have described the phytochemical composition of the essential oil and organic extracts from A. subhirsitum plant organs (bulbs, flowers, and leaves). Our team has demonstrated that methanolic extract from hairy garlic (bulbs) helps with antioxidant, anti-inflammatory, and anticancer claims, and can inhibit tumor angiogenesis in a murine model of skeletal metastases [16,20]. Nencini and colleagues reported the antioxidant activities of aged 15% aqueous ethanol extract from Italian A. subhirsutum leaves, flowers, and bulbs [21]. Emir and colleagues reported the cholinesterases and tyrosinase inhibition activities of Turkish A. subhirsutum (methanolic extract from bulbs and aerial parts) [18].
Hence, the present work was the first to quantitatively analyze the phytochemical constituents of the aqueous extract obtained from of A. subhirsutum bulbs. Additionally, the determination of its antioxidant, antimicrobial, anti-quorum sensing, antibiofilm, cytotoxicity, and antiviral properties with the aqueous extract was also carried out. Moreover, to screen potential drugs/molecules from the above extract, pharmacokinetics and toxicological prediction were also performed.
2. Results
2.1. Phytochemical Composition of A. subhirsutum L. Aqueous Extract
The aqueous extract from the hairy garlic bulbs was screened for the presence of different classes of biomolecules. Results showed that hairy garlic aqueous extract is a rich source of saponins, terpens, flavonols/flavonones, flavonoids, and fatty acids.
Table 1 summarizes the tentative identification of phytoconstituents in the tested extract by using the GC–MS technique. Fifteen compounds, belonging to different chemical classes, were identified. 5-hydroxymethylfurfural (37.04%), methyl methanethiolsulfonate (21.33%), furfural (7.64%), beta-D-glucopyranose, 1,6-anhydro- (6.17%), and 1,6-anhydro-beta-D-glucofuranose (3.6%) were the dominant compounds, followed by trisulfide, di-2-propenyl (2.70%), and diallyl disulfide (1.93%). Their chemical formula and molecular weight are listed in Table 1.
Table 1.
Phytochemical composition of aqueous extract of A. subhirsutum L. (bulbs) using the GC–MS technique.
| N° | Identified Compound Name | RT [min] | Area (%) | Molecular Weight (g/mol) |
Formula |
|---|---|---|---|---|---|
| 1 | Methyl methanethiolsulfonate | 1.462 | 21.33 | 126.20 | C2H6O2S2 |
| 2 | Propanoic acid, 2-oxo-, methyl ester | 2.201 | 1.29 | 102.09 | C4H6O3 |
| 3 | Furfural | 3.131 | 7.64 | 96.08 | C5H4O2 |
| 4 | Diallyl disulfide | 6.725 | 1.93 | 146.27 | C6H10S2 |
| 5 | 2,4,5-trimethyl-1,3-dioxolane | 7.681 | 2.48 | 116.16 | C6H12O2 |
| 6 | 5-hydroxymethylfurfural | 8.857 | 37.04 | 126.11 | C6H6O3 |
| 7 | 1H-azonine, octahydro-1-nitroso- | 9.206 | 4.09 | 156.23 | C8H16N2O |
| 8 | Trisulfide, di-2-propenyl | 10.093 | 2.70 | 178.34 | C6H10S3 |
| 9 | Beta-D-fructofuranosyl alpha-D-glucopyranoside | 11.731 | 4.40 | 342.30 | C12H22O11 |
| 10 | Beta-D-glucopyranose, 1,6-anhydro- | 12.463 | 6.17 | 288.25 | C12H16O8 |
| 11 | Beta-D-glucofuranose, 1,6-anhydro- | 13.638 | 3.60 | 162.14 | C6H10O5 |
| 12 | Palmitic acid, methyl ester | 17.342 | 3.32 | 270.45 | C17H34O2 |
| 13 | n-hexadecanoic acid | 17.664 | 1.46 | 256.42 | C16H32O2 |
| 14 | 9,12-octadecadienoic acid, methyl ester | 18.974 | 1.32 | 294.47 | C19H34O2 |
| 15 | Octadecanoic acid, methyl ester | 19.270 | 1.24 | 298.5 | C19H38O2 |
The chromatogram obtained showed, respectively, the peaks of the identified molecules with their chemical structure (Supplementary Material S1).
2.2. Biological Activities of A. subhirsutum L. Aqueous Extract
2.2.1. Cytotoxicity Evaluation
Results obtained using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] coloremetric method against the African green monkey kidney cell lines (VERO cell lines) showed that hairy garlic aqueous extract was not toxic as the 50% cytotoxic concentration (CC50%) was higher than 30,000 µg/mL.
2.2.2. Antimicrobial Activities
The hairy garlic aqueous extract was tested against a large collection of Gram-positive and Gram-negative bacteria, yeasts, molds, and coxsakievirus B3 (CVB3), and herpes simplex virus type 2 (HSV-2). Results of the antiviral activity showed that A. subhirsutum aqueous extract was not active against the two tested virus (CVB3) and (HSV-2).
Results of the antibacterial and antifungal activities of the hairy garlic aqueous extract using both disc diffusion and microdilution assays are summarized in Table 2.
Table 2.
Growth inhibition zone, as well as MIC, MBC, and MFC/MIC values obtained for all microorganisms tested using disc diffusion and microdilution assays tested using hairy garlic aqueous extract.
| Code | Strains |
A. subhirsutum L. Aqueous Extract (10 µL/disc; 100 mg/mL) |
Ampicillin (10 µL/disc; 50 mg/mL) |
|||
|---|---|---|---|---|---|---|
| Mean ± SD * (mm) | MIC | MBC | MBC/MIC | Mean ± SD (mm) | ||
| B1 | E. coli ATCC 35218 | 6.00 ± 0.00 fB | 12.5 | 50 | 4 | 7.00 ± 0.00 dA |
| B2 | P. aeruginosa ATCC 27853 | 11.67 ± 0.57 dA | 6.25 | 50 | 8 | 7.33 ± 0.57 dB |
| B3 | P. mirabilis ATCC 29245 | 7.00 ± 0.00 eA | 12.5 | 100 | 8 | 6.33 ± 0.57 dA |
| B4 | K. pneumoniae ATCC 27736 | 6.00 ± 0.00 fA | 12.5 | 100 | 8 | 6.66 ± 0.57 dA |
| B5 | P. mirabilis | 6.00 ± 0.00 fB | 12.5 | 100 | 8 | 21.00 ± 1.00 aA |
| B6 | S. sciuri | 6.00 ± 0.00 fB | 12.5 | 100 | 8 | 7.00 ± 0.00 dA |
| B7 | S. pyogens | 15.66 ± 0.57 aA | 6.25 | 50 | 8 | 16.00 ± 1.73 bA |
| B8 | P. aeruginosa | 6.00 ± 0.00 fA | 12.5 | 100 | 8 | 6.66 ± 0.57 dA |
| B9 | S. aureus MDR | 15.66 ± 0.57 aA | 6.25 | 50 | 8 | 7.33 ± 0.57 dB |
| B10 | E. cloacae | 13.33 ± 0.57 cA | 6.25 | 50 | 8 | 6.66 ± 0.57 dB |
| B11 | S. paucimobilis | 6.00 ± 0.00 fB | 12.5 | 100 | 8 | 7.66 ± 0.57 dA |
| B12 | A. baumannii | 14.33 ± 0.57 bA | 6.25 | 50 | 8 | 13.33 ± 0.57 cB |
| Code | Strains |
A. subhirsutum L. Aqueous Extract
(10 µL/disc; 100 mg/mL) |
Amphotericin B
(10 µL/disc; 10 mg/mL) |
|||
| Mean ± SD * (mm) | MIC | MFC | MFC/MIC | Mean ± SD (mm) | ||
| Y1 | C. albicans ATCC 10231 | 6.66 ± 0.57 cB | 3.12 | 50 | 16 | 22.66 ± 1.15 aA |
| Y2 | C. neoformans ATCC 14116 | 6.33 ± 0.57 cB | 3.12 | 50 | 16 | 15.33 ± 0.57 bA |
| Y3 | C. vaginalis | 15.66 ± 0.57 aA | 1.56 | 25 | 16 | 6.66 ± 0.57 dB |
| Y4 | C. albicans | 6.00 ± 0.00 cB | 6.25 | 50 | 8 | 12.33 ± 0.57 cA |
| M1 | A. fumigatus ATCC 204305 | 10.33 ± 0.57 bB | (−) | (−) | (−) | 15.00 ± 1.00 bA |
| M2 | A. niger | 6.00 ± 0.00 cA | (−) | (−) | (−) | 6.00 ± 0.00 dA |
* Inhibition zone around the discs impregnated with A. subhirsutum L. aqueous extract expressed as the mean of three replicates (mm ± SD). SD: standard deviation. MIC: minimal inhibitory concentration (mg/mL). MBC: minimal bactericidal concentration (mg/mL). MFC: minimal fungicidal concentration (mg/mL). a–f,A,B: Each value represents the average of 3 repetitions. The means followed by the same letters are not significantly different at p = 0.05 based on Duncan’s multiple-range test. Small letters are used to compare aqueous extract and antibiotic means between different strains, while capital letters are used to compare means between aqueous extract and antibiotics for the same strain. (−): Not tested.
The mean diameter of the growth inhibition zone (mm ± SD) varied from 6.00 ± 0.00 to 15.66 ± 0.57. Using the scheme proposed by Parveen et al. (2010), our extract had low (1–6 mm) to high (11–15 mm) activity against all tested Gram-positive and Gram-negative bacteria. Most of tested microorganisms were more sensitive to the tested extract as compared to the standard antibiotic used (ampicillin). Using the microdilution assay, MIC values ranged from 6.25 to 12.5 mg/mL and concentrations around 50–100 mg/mL could kill the tested microorganisms (MBCs values). The MBC/MIC ratio ranged from 4 to 8, highlighting the bacteriostatic activity of the tested extract on all tested microorganisms. This extract was also active against yeast and mold strains at varying degrees. The Candida vaginalis strain was the most sensitive to hairy garlic extract with a mean diameter of about (15.66 ± 0.57 mm) in the growth inhibition zone. MIC values ranged from 1.62 to 6.25 mg/mL and MFCs values ranged from 25 to 50 mg/mL. The tested aqueous extract exhibited fungistatic activity against all Candida spp. strains tested (MFC/MIC ratio > 4).
2.2.3. Antioxidant Activities
The quantitative analysis of tannins, phenols, and flavonoids demonstrated that hairy garlic contains large quantities of flavonoids (231 ± 0.022 mg QE/g extract), followed by tannins (159 ± 0.006 mg TAE/g extract) and phenols (4 ± 0.004 mg GAE/g extract). The results of the free radical scavenging activities of A. subhirsutum L. aqueous extract, as compared to ascorbic acid and butylated hydroxyl-toluene (BHT), are reported in Table 3. The concentrations needed for scavenging 50% of radicals (IC50%) using hairy garlic aqueous extract were as follows: 1 mg/mL for DPPH and FRAP assays (respectively), (0.698 ± 0.107) mg/mL for the ABTS test, and (0.811 ± 0.036) mg/mL for the β-carotene experiment.
Table 3.
Antioxidant activities of aqueous extract of A. subhirsutum L. (bulbs), as compared to ascorbic acid and BHT.
| Test Systems | Hairy Garlic Extract | (BHT) | (AA) |
|---|---|---|---|
| Total flavonoids content (mg QE/g extract) | 231 ± 0.022 | − | − |
| Total tannins content (mg TAE/g extract) | 159 ± 0.006 | − | − |
| Total phenols content (mg GAE/g extract) | 4 ± 0.004 | − | − |
| DPPH IC50 (mg/mL) | 1 a | 0.023 ± 3 × 10−4 b | 0.022 ± 5 × 10−4 b |
| ABTS IC50 (mg/mL) | 0.698 ± 0.107 a | 0.018 ± 4 × 10−4 b | 0.021 ± 0.001 b |
| β-carotene IC50 (mg/mL) | 0.811 ± 0.036 a | 0.042 ± 3.5 × 10−3 b | 0.017 ± 0.001 c |
| FRAP IC50 (mg/mL) | 1 a | 0,05 ± 0.003 c | 0.09 ± 0.007 b |
BHT: butylated hydroxytoluene, AA: ascorbic acid. The letters (a–c) indicate a significant difference between the different antioxidant methods according to Duncan’s test (p < 0.05). (−): Not tested.
2.2.4. Anti-Quorum Sensing and Antibiofilm Activities
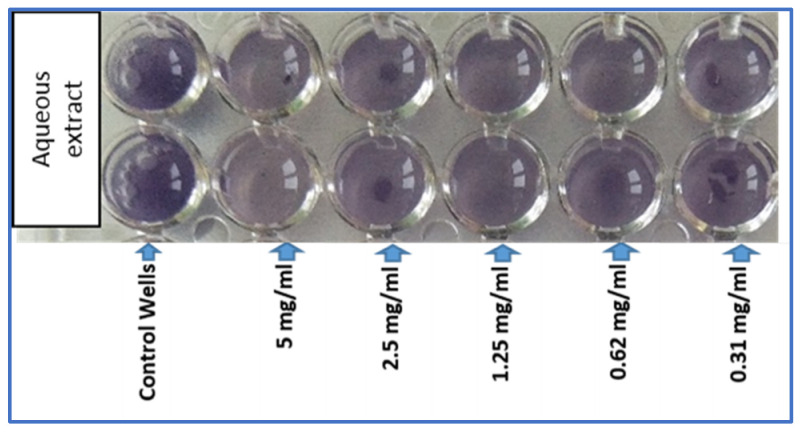
The ability of A. subhirsutum aqueous extract to inhibit the production of violacein, a pigmented molecule secreted by C. violaceum when bacterial concentration is reached (quorum sensing), was tested using the microtiter plate assay (mutant-C. violaceum CV026) and the Petri dishes test using Luria–Bertani agar medium (wild type C. violaceum ATCC 12472). The results showed that the tested extract was able to inhibit the production of violacein by the mutant C. violaceum strain in a concentration-dependent manner (Figure 1). In fact, the percentage of violacein production was inhibited by (24.05 ± 0.68)% at 2.5 mg/mL and about (37.43 ± 0.85)% at 5 mg/mL. No inhibition of violacein production was recorded at low concentrations of hairy garlic extract (1.25 and 0.625 mg/mL).
Figure 1.
Qualitative method for the determination of the effect of different concentrations of A. subhirsutum aqueous extract on violacein production by C. violaceum CV026.
Using LB Petri dish agar plates, the tested extract was able to inhibit the production of violacein by the wild-type starter strain (C. violaceum ATCC 12472). Furthermore, the diameter of growth inhibition zone ranged from (8 ± 1) mm at 1.25 mg/mL to (13 ± 0.5) mm at 5 mg/mL. All these data are summarized in Table 4.
Table 4.
Violacein inhibition anti-quorum sensing activities of A. subhirsutum aqueous extract. (−): No activity.
| Test | A. subhirsutum L. Aqueous Extract (mg/mL) | |||
|---|---|---|---|---|
| 5 | 2.5 | 1.25 | 0.625 | |
| Violacein inhibition (%) | 37.43 ± 0.85 | 24.05 ± 0.68 | (−) | (−) |
| Anti-quorum sensing activity (mm) | 13 ± 0.5 | 10 ± 1 | 8 ± 1 | (−) |
In addition, two virulence properties controlled by the quorum-sensing system in the P. aeruginosa PAO1 strain (swarming and swimming) were inhibited at varying degrees when different concentrations of hairy garlic aqueous extract were used. In fact, the percentage of the inhibition of swarming activity ranged from (8.93 ± 0)% at 50 µg/mL to (23.66 ± 0.5)% at 100 µg/mL. Furthermore, the swimming activity was reduced by (13.67 ± 1)% at 100 µg/mL of the hairy garlic aqueous extract (Table 5).
Table 5.
Anti-swarming and anti-swimming activities of A. subhirsutum extracts.
| Tests | 100 | 75 | 50 |
|---|---|---|---|
| Swarming inhibition (%) | 23.66 ± 0.5 | 16.96 ± 1 | 8.93 ± 0 |
| Swimming inhibition (%) | 13.67 ± 1 | (−) | (−) |
Concentration is expressed as µg/mL; (−): no activity; %: percentage.
The antibiofilm activity of the tested extract was experimented on four bacteria and two Candida spp. strains at different concentrations, ranging from MIC/16 to MIC (from 0.312 to 10 mg/mL). Table 6 summarizes the percentage of biofilm inhibition when different concentrations of extract were used.
Table 6.
Anti-biofilm results (inhibition %) of A. subhirsutum L. aqueous extract tested against Gram-positive and Gram-negative bacteria and yeast strains.
| Microorganisms Tested | Concentration Used | A. subhirsutum L. Aqueous Extract |
|---|---|---|
| S. aureus ATCC 25923 | MIC = 10 mg/mL | 56.21 ± 2.55 b |
| MIC/2 = 5 mg/mL | 20.50 ± 1.78 b | |
| MIC/4 = 2.5 mg/mL | 2.82 ± 0.13 c | |
| L. monocytogenes ATCC 7644 | MIC = 10 mg/mL | 12.18 ± 1.24 e |
| E. coli ATCC 25922 | MIC = 10 mg/mL | 18.50 ± 1.35 d |
| S. typhi ATCC 14028 | MIC = 10 mg/mL | 32.97 ± 2.56 c |
| MIC/2 = 5 mg/mL | 18.49 ± 1.84 c | |
| MIC/4 = 2.5 mg/mL | 5.24 ± 0.32 b | |
| C. albicans ATCC 10239 | MIC = 10 mg/mL | 62.48 ± 5.50 a |
| MIC/2 = 5 mg/mL | 35.40 ± 4.25 a | |
| MIC/4 = 2.5 mg/mL | 15.23 ± 2.52 a | |
| C. tropicalis ATCC 13803 | MIC = 5 mg/mL | 54.81 ± 4.08 b |
| MIC/2 = 2.5 mg/mL | 17.95 ± 2.20 c | |
| MIC/4 = 1.25 mg/mL | 5.20 ± 0.62 b |
Each value represents the average of 3 repetitions. The means followed by the same letters are not significantly different at p = 0.05 based on Duncan’s multiple-range test. Small letters are used to compare the means between strains for each concentration of aqueous extract.
Interestingly, at an MIC value of (10 mg/mL), the percentage of biofilm inhibition depended on the tested strain and increased from (12.18 ± 1.24)% for L. monocytogenes ATCC 7644, (18.50 ± 1.35)% for E. coli ATCC 25922, (32.97 ± 2.56)% for S. typhi ATCC 14028, and (56.21 ± 2.55)% for S. aureus ATCC 25923. The fungal biofilm was also inhibited at low concentrations. In fact, 1.25 mg/mL of hairy garlic extract inhibited the ability of C. albicans ATCC 10239 and C. tropicalis ATCC 13803 by (5.20 ± 0.62)% and (15.23 ± 2.52)%, respectively. The highest inhibition was recorded at an MIC value of (10 mg/mL of aqueous extract) with a percentage of inhibition at about (62.48 ± 5.50)% for C. albicans ATCC 10239 and (54.81 ± 4.08)% for C. tropicalis ATCC 13803. Based on Duncan’s multiple-range test, there was no significant difference between the percentage of biofilm inhibition of the two Candida strains tested at 10 mg/mL.
2.2.5. Pharmacokinetic Properties and Toxicity Profile Prediction
During the time of the preclinical analysis trial in drug discovery and development, the assessment of absorption, distribution, metabolism excretion, and toxicity (ADMET) are very crucial for attractive molecules to possess the best chance to become an effective drug. Hence, based on ADME analysis, the identified phytocompounds from A. subhirsutum water extract were predicted for their pharmacokinetics, drug-likeness, and medicinal chemistry friendliness using the SwissADME web tool. As shown in (Table 7), all selected phytoconstituents did not violate the Lipinski’s rule of five; therefore, they seem to be passed orally with suitable bioavailability scored at 0.55. They exhibited high gastrointestinal (GI) absorption with eight compounds being able to cross the blood-brain–barrier (BBB) permeant; this revealed that they have low to no central nervous system (CNS) side effects. Only ten compounds were found in the substrate for permeability glycoprotein (P-gp), meaning that they possess very little chance to efflux out of the cell. Moreover, compounds 1–7 did not inhibit all the tested cytochrome P450 isoenzymes which played a fundamental role in the biotransformation of drugs through O-type oxidation reactions.
Table 7.
ADMET properties of the identified compounds.
| Entry | Compounds * | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Physicochemical Properties/Lipophilicity/Drug-likeness | ||||||||||||||
| Molecular Weight | 126.20 | 102.09 | 96.08 | 146.27 | 116.16 | 126.11 | 156.23 | 178.34 | 288.25 | 162.14 | 270.45 | 256.42 | 294.47 | 298.50 |
| Num. heavy atoms | 6 | 7 | 7 | 8 | 8 | 9 | 11 | 9 | 11 | 19 | 18 | 21 | 21 | 21 |
| Num. arom. heavy atoms | 0 | 0 | 5 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fraction Csp3 | 1.00 | 0.50 | 0.00 | 0.33 | 1.00 | 0.17 | 1.00 | 0.33 | 1.00 | 0.94 | 0.94 | 0.74 | 0.95 | 0.95 |
| Num. rotatable bonds | 1 | 2 | 1 | 5 | 0 | 2 | 1 | 6 | 0 | 15 | 14 | 15 | 17 | 17 |
| Num. H-bond acceptors | 2 | 3 | 2 | 0 | 2 | 3 | 2 | 0 | 5 | 2 | 2 | 2 | 2 | 2 |
| Num. H-bond donors | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 |
| Molar refractivity | 28.28 | 22.83 | 24.10 | 45.19 | 31.01 | 30.22 | 50.00 | 52.78 | 32.38 | 85.12 | 80.80 | 93.78 | 94.73 | 94.73 |
| TPSA (Ų) | 67.82 | 43.37 | 30.21 | 50.60 | 18.46 | 50.44 | 32.67 | 75.90 | 79.15 | 26.30 | 37.30 | 26.30 | 26.30 | 26.30 |
| Lipinski’s rule | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Pharmacokinetics/Toxicity prediction | ||||||||||||||
| GI absorption | High | High | High | High | High | High | High | High | High | High | High | High | High | High |
| BBB permeant | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | No | No |
| P-gp substrate | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | No | No | Yes | Yes | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| AMES toxicity | Yes | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Hepatotoxicity | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| hERG I/II inhibitors | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
* Compound name is the same as listed in Table 1.
The AMES toxicity and hepatotoxicity of the parameters of hERG I/II inhibitors were evaluated using the pkCSM online server which predicted whether the designed new molecules were toxic. Based on the predictive results (Table 7), except the compound 1, none of the others were expected to present any toxicity problems.
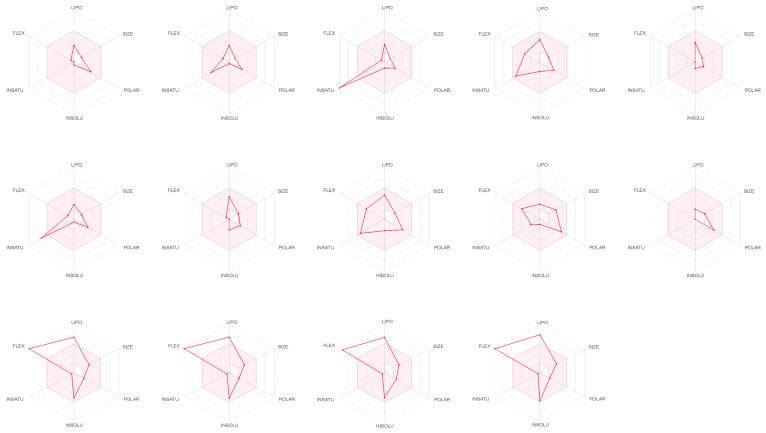
An estimation of drug-likeness properties based on bioavailability radar (Figure 2) remains a powerful tool to understand identified compounds, as well as their lipophilicity, size, polarity, solubility, saturation, and flexibility behaviors. In addition, as shown in Figure 2, most of the identified compounds fit totally in the pink area, signifying their good predicted oral bioavailability.
Figure 2.
Bioavailability radar of identified compounds based on the physicochemical indices ideal for oral bioavailability. LIPO, lipophilicity: −0.7 < XLOGP3 < þ 5; SIZE, molecular size: 150 g/mol < molecular weight < 500 g/mol; POLAR, polarity: 20 Å2 < TPSA (Topological Polar Surface Area) < 130 Å2; INSOLU, insolubility: 0 < Log S (Insolubility in water: ESOL) < 6; INSATU, Insaturation: 0.25 < Fraction of carbons in the sp3 hybridization < 1; FLEX, flexibility: 0 < the number of rotatable bonds < 9. The colored zone is the suitable physicochemical space for oral bioavailability.
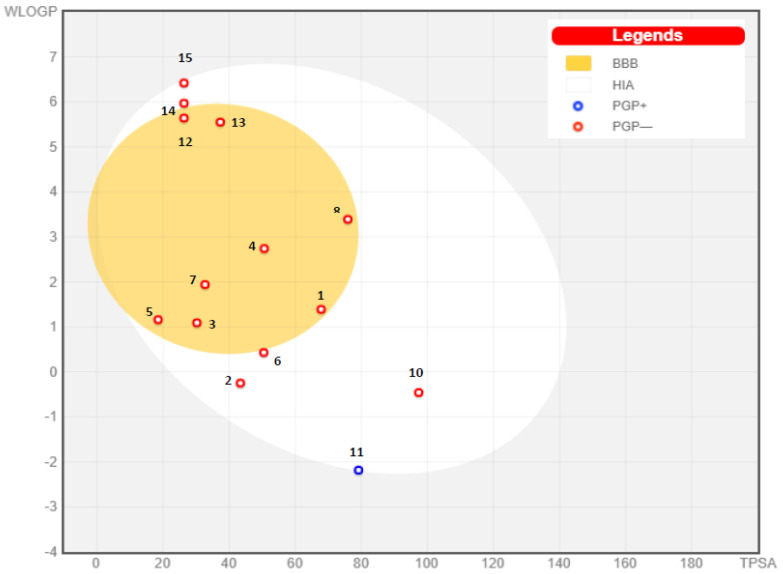
To know more about both passive gastrointestinal absorption (HIA) and the BBB penetrating effect as a function of the position of the molecules in the WLOGP-versus-TPSA referential, the boiled egg model (Figure 3) has been established for the top ADME compounds. The results clearly indicate that compounds 2, 6, 10, 11, 14, and 15 were in the white zone, thus indicating the high probability of being passively absorbed by the gastrointestinal tract with only 11 appearing as a blue point, which was predicted as a substrate of the PGP+. On the other hand, compounds 1, 3, 4, 5, 7, 8, 12, and 13 were in the in the yolk region, which reflects their high probability to permeate through BBB to access CNS, and also appeared in the red point, suggesting that the substrate of the p-glycoprotein is actively effluxed by PGP+.
Figure 3.
(BOILED–Egg) model of the selected compound. The names of the compounds are listed in Table 1.
3. Discussion
In the present study, we were the first to investigate the chemical composition of A. subhirsutum (bulbs) aqueous extract using the GC–MS technique. We reported the identification of 15 bioactive compounds belonging to different known classes of bioactive molecules. In fact, 5-hydroxymethylfurfural (37.04%), methyl methanethiolsulfonate (21.33%), furfural (7.64%), trisulfide, di-2-propenyl (2.70%), and diallyl disulfide (1.93%) were identified and compared to published data, as reviewed in Table 8.
Table 8.
Review of the phytochemical compounds identified in A. subhirsutum L. (bulbs and aerial parts) extracts from different origins.
| Organ Tested/Origin | Solvent and Technique Used |
Identified Molecules | Reference |
|---|---|---|---|
| Fresh bulbs (Hail, Saudi Arabia) |
Methanol HR-LCMS ** |
Tripeptides: Tyr Trp Phe, Asn Asn Asn, Cys Tyr Trp, Thr Asp Asn, Cys Tyr Trp, Phe Glu, Asp Arg Tyr, Val Ser Cys, Asn Gln Ala, Val Glu Asp, Gly Tyr Lys, Lys Arg Lys; Dipeptides: Pro Leu, His Asp, Glu Thr, Phe Pro. Bioactive compounds: methyl N-(amethylbutyryl) glycine; bis(2-hydroxypropyl)amine; cepharanthine; 2-methylene-5-(2,5dioxotetrahydrofuran-3-yl)-6-oxo--10,10-dimethylbicyclo [7:2:0] undecane; (22S)-1alpha,22,25-trihydroxy-26,27-dimethyl-23,23,24,24-tetradehydro-24ahomovitamin D3/(22S)-1al; L-4-hydroxy-3-methoxy- amethylphenylalanine N-(2-fluro-ethyl) arachidonoyl amine; 1-nonadecanoyl-2- (5Z,8Z,11Z,14Z,17Zeicosapentaenoyl)-sn-glycerol; TG(16:1(9Z)/17:2(9Z,12Z)/20: 5(5Z,8Z,11Z,14Z,17Z)) [iso6]; L-4-hydroxy-3-methoxy-amethylphenylalanine; 11 alpha-acetoxykhivorin; methyl gamboginate; dihydrodeoxystreptomycin; 6 alpha-hydroxy castasterone; C16 sphinganine; 3beta,7alpha,12alpha-trihydroxy-5alpha-cholestan-26-oic acid; 4-oxomytiloxanthin; sebacic acid; tuberonic acid; 6-deoxocastasterone; linolenoyl lysolecithin; 19-amino-16-hydroxy-16-oxido-10-oxo-11,15,17-trioxa-165-phosphanonadecan-13-ylundecanoate GPETn(10:0/11:0) [U]; 3beta,7alpha,12alpha-trihydroxy-5alpha-cholestan- 26-oic acid, N-(2-hydroxyethyl) stearamide; 2,2-difluoro-hexadecanoic acid. | [16] |
| Dried pulverized flowering aerial parts/Bulbs (Palermo, Italy) |
Absolute ethanol (≥99.8%) LC-ESI-MSn+ |
Sulfur compounds: allicin, gamma-glutamyl (S)-allylcysteine, gamma-glutamyl-S-methylcysteine, gamma-glutamyl-S-trans-propenyl cysteine, alliin, cycloalliin. Flavonoids and polyphenols: methoxy quercetin trisaccharide isomer, methoxy quercetin isomer, quercetin, methoxy quercetin trisaccharide isomer, luteolin, methoxy quercetin isomer, glucosyl gallate, kaempferol, methoxy quercetin isomer, 3,7-dimethylquercetin, tamarixetin (3,30,5,7-Tetrahydroxy-40-methoxyflavanone), 5,3´,4´-T-trihydroxy-3-methoxy-6,7-methylenedioxy flavone. Amide phenylpropanoid derivatives: coumaroyl-tyramine, N-trans-feruloyl-tyramine, coumaroyl-octopamine, N-trans-feruloyl-3-methoxytyramine. | [17] |
| Air-dried powdered aerial parts/bulbs Menderes (İzmir/Turkey) |
Methanol LC-ESI-MSn |
Phenolic compounds: benzoic acid; 3-hydroxybenzoic acid; 4-hydroxybenzoic acid; p-coumaric acid; vanillic acid; gallic acid; ferulic acid; syringic acid; aidzein; chrysin; kaempferol; luteolin; fisetin; morin; quercetin; 3-O-methylquercetin; isorhamnetin; galangin; myricetin; vitexin; hesperidin; 3-hydroxyflavone; naringenin; genistein; phenyl acetate; catechol; (+)-catechin; (−)-epicatechin; (−)-epigallocatechin gallate. | [18] |
LC-ESI-MSn: liquid chromatography electrospray ionization tandem mass spectrometric; HR-LCMS **: high-resolution liquid chromatography mass spectroscopy.
By using the LC-MS technique, we reported the identification of 16 small peptides (5 dipeptides and 11 tripeptides), and 25 phytoconstituents dominated by sebacic acid, 11alpha-acetoxykhivorin, cepharanthine, methyl gamboginate, hexadecasphinganine, 4-Oxomytiloxanthin, and linolenoyl lysolecithin from hairy garlic methanolic extract [16]. In 2020, Sut and colleagues [17] reported the identification of several bioactive compounds from the aerial parts and bulbs of A. subhirsutum L. (ethanolic extract) collected from Padova (Italy). The ethanolic extract from the dried pulverized bulbs was dominated by alliin (29.51 ± 0.04 mg/g), allicin (26.40 ± 0.02 mg/g), gamma-glutamyl (S)-allylcysteine (10.97 ± 0.02 mg/g), luteolin (20.19 ± 0.03 mg/g), methoxy quercetin isomer (22.19 ± 0.02 mg/g), glucosyl gallate (21.61 ± 0.0 mg/g), and N-trans-feruloyl-tyramine (41.88 ± 0.10 mg/g). In addition, Emir and colleagues [18] reported the identification of 30 phenolic compounds from the aerial parts of A. subhirsutum L. methanolic extract by using LC-ESI-MS/MS technique. The major phenolic acids identified were: benzoic acid (39.2 ± 2.83; 146.7 ± 0.95) µg/mL; 3-hydroxybenzoic acid (518.6 ± 1.98; 430.1 ± 2.63) µg/mL; 4-hydroxybenzoic acid (976.7 ± 3.44; 314.2 ± 1.78) µg/mL; p-coumaric acid (1700.8 ± 1.52; 1042.4 ± 2.97) µg/mL; vanillic acid (903.8 ± 2.31; 621.6 ± 1.87) µg/mL; gallic acid (44.5 ± 0.93; 52.3 ± 2.21) µg/mL; ferulic acid (787.2 ± 1.18; 1352.0 ± 3.16) µg/mL; and genistein (130.6 ± 1.42; 159.3 ± 2.76) µg/mL, respectively, for the methanolic extract from air-dried and powdered bulbs and aerial parts [18]. In 2018, Küçük and colleagues [19] were the first to study the headspace volatiles from A. subhirsutum L. crashed bulbs from Turkey using the gas chromatography–mass spectrometry technique. They reported the identification of six compounds namely allyl methyl disulfide (41.0%), diallyl disulfide (20.7%), dimethyl sulfide (15.3%), methyl (methylthiol) methyl disulfide (2.0%), methyl trans-propenyl disulfide (1.9%), and allyl methyl trisulfide (1.4%).
Most of the identified compounds in hairy garlic aqueous extract were previously described in the composition of different Allium species, as summarized in Table 9.
Table 9.
Review of the distribution of some identified bioactive compounds in some Allium plant species.
| Bioactive Molecule | Allium Species/Variety | References |
|---|---|---|
| Methyl methanethiolsulfonate | Allium hirtifolium | [25] |
| Allium hooshidaryae | [26] | |
| Allium sativum | [27] | |
| Allium ursinum | [28] | |
| Furfural | Allium fistulosum | [29] |
| Black garlic | [30] | |
| Diallyl disulfide | Allium hookeri | [31] |
| Black garlic | [30,32] | |
| Allium sativum | [27] | |
| Allium tuncelianum | [33] | |
| 2,4,5-trimethyl-1,3-dioxolane | Allium hirtifolium | [25] |
| 5-hydroxymethylfurfural | Allium hirtifolium | [25] |
| Black garlic | [32,34] | |
| Allium fistulosum | [29] | |
| Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] | |
| Trisulfide, di-2-propenyl | Allium hookeri | [31] |
| Black garlic | [30] | |
| Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] | |
| Allium tuncelianum | [33] | |
| Beta-D-fructofuranosyl alpha-D-glucopyranoside | Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] |
| Palmitic acid, methyl ester | Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] |
| n-hexadecanoic acid | Allium hirtifolium | [25] |
| Allium fistulosum | [29] | |
| Allium willeanum | [36] | |
| Allium sativum (varieties Taicangbaip, Hongqixing, Ershuizao and single clove) | [35] | |
| 9,12-octadecadienoic acid, methyl ester | Allium hirtifolium | [25] |
| Allium ampeloprasum, var. holmens | [37] | |
| Octadecanoic acid, methyl ester | Allium fistulosum | [29] |
In terms of the phytochemical composition, our results were similar to those obtained by Saoudi and colleagues in 2021 [20]. This team quantified the total phenols and flavonoids in A. subhirsutum cloves (methanolic extract and oil) collected from Sfax (Tunisia). They founded that the methanolic extract was a rich source of tannins, phenols, and flavonoids (41.7 ± 3.4), as compared to the corresponding oil. The difference in the phytochemical composition can be attributed to the solvent used. In fact, using the same plant species from the same origin (Hail, Saudi Arabia) and methanol as eluent, our team reported different amounts of these classes of compounds. The origin of plant samples also affects the quantity of phenolic and flavonoid contents. In fact, Emir and colleagues [18] reported that the total phenolic content of A. subhirsutum methanolic extract from the aerial parts was about (13.3 ± 1.7 mg of quercetin equivalent/g of dry plant extract) as compared to bulbs (15.8 ± 0.9 mg of quercetin equivalent/g of dry plant extract). On the other hand, the quantity of flavonoids was higher in bulbs (5.7 ± 0.8 mg of quercetin equivalent/g of dry plant extract) as compared to the aerial parts (3.6 ± 0.3 mg of quercetin equivalent/g of dry plant extract). Interestingly, Nencini and colleagues [21] confirmed that phenolics and flavonoids decreased in the aged hydroethanolic extract (up to 20 months) of different organs from A. subhirsutum. In fact, the polyphenol levels in aged hairy garlic extract were about (0.39 ± 0.01 mg GAE/g) in bulbs, (1.22 ± 0.01 mg GAE/g) in leaves, and (1.09 ± 0.02 mg GAE/g) in flowers.
No scientific report discussed the anti-quorum sensing and the antibiofilm activities of A. subhirsutum plant extracts. Interestingly, our extract was able to modulate the secretion of violacein by both C. violaceum mutant and wild type. The same extract inhibited the swarming and swimming motility mode of P. aeruginosa PAO1 in a concentration-dependent manner. Previous reports have focused on extracts from A. cepa and A. sativum plant species. Results showed that sulfur compounds (Ajoene, Iberin) from garlic and flavonoids (Quercetin) from red onion modulated the production of bioluminescence in Vibrio harveyi, violacein in C. violaceum, and pyocyanin/proteases/elastases and swarming motility in P. aeruginosa [38]. Allium cepa (95% methanolic) extract was also able to inhibit the production of the green pigment (Pyocyanin) by P. aeruginosa PAO1 and to reduce its motility by reducing swimming, twitching, and swarming abilities [39]. The same extract was able to reduce the production of violacein on agar medium with a mean diameter of about 10 mm in the growth inhibition zone and to prevent the formation of biofilm formed by P. aeruginosa (Isolate PA14) using the tube assay method [40].
A. sativum (methanolic and ethanolic) extracts were able to inhibit the biofilm formed by six pathogenic bacteria including S. aureus, Bacillus cereus, Streptococcus pneumoniae, P. aeruginosa, E. coli, and K. pneumoniae in a concentration-dependent manner [41]. Bhatwalkar and colleagues in 2019 [42] reported that the fresh garlic extract at 4% significantly inhibited the biofilm formed by Shiga-toxin-producing E. coli (STEC) strains. More recently, Caputo et al., [43] reported the antibiofilm activities of two landraces (Irsina and Contursi) of A. ampeloprasum var. holmense from south Italy (methanolic extract from bulbs and aerial parts) which were tested against Listeria monocytogenes ATCC 7644, entero-hemorrhagic E. coli DSM 8579, P. aeruginosa DSM 50071, Pectobacterium carovotorum DSM 102074, and S. aureus ATCC 25923.
The reported biological properties of the tested extract can be attributed to the presence of many phytocompounds with promising activities. In fact, it is well documented that the main identified compound in hairy garlic extract, 5-hydroxymethylfurfural, possessed antioxidant and antiproliferative activities [44], as well as anti-ischemic and anti-tyrosine enzyme effects, improving blood rheology and affecting the role of glycyrrhizin metabolism [45]. It has been demonstrated that this compound (5-hydroxymethylfurfural) can protect human vein epidermal cell against H2O2 and glucose and improve acute liver injury in mice [46].
Additionally, methyl methanethiolsulfonate which is produced in different amounts by Alliaceae members is an anti-oomycete agent with antimicrobial and antimutagenic characters [47]. In addition, this sulfur compound is known to inhibit colon tumor [48]. More recently, Vijayakumar and Ramanathan [49] demonstrated that 5-hydroxymethylfurfural showed interesting anti-quorum sensing and antibiofilm activities against C. violaceum, Streptococcus pyogenes, S. mutans, S. aureus, and S. epidermidis. In fact, at 100 μg/mL, this compound inhibited the production of violacein by 87% and reduced the biofilm formation by S. mutans and S. epidermidis strains by 86% and 79%, respectively. The streptococcal ad staphylococcal biofilm formation was also inhibited with high percentage at 125 μg/mL (up to 83% for S. pyogens, and 82% for S. aureus).
Furfural, a natural furan occurring compound, is known to possess antityrosinase and antimicrobial activities against Bacillus subtilis with an antimicrobial zone between 16 and 20 mm at 1.4 µM, MIC/MBC values about 0.027 µM [50]. This compound (furfural) was also active against Salmonella bacteria with an antimicrobial zone less than 15 mm at concentrations ranging from 0.35 to 1.4 µM, and MIC/MBC values of about 0.029 µM and 0.121 µM, respectively [50]. In addition, the identified sulfur compounds diallyl disulfide and diallyl trisulfide are known to be potent phytoconstituents for the prevention and treatment of several human diseases, such as endocrine system diseases, cardiovascular diseases, neurological diseases, infectious diseases, and cancerous diseases [51,52,53,54].
Similarly, hexadecenoic acid identified in hairy garlic aqueous extract is known to exhibit anti-tumoral, antimicrobial, antioxidant, anti-cholesterol, and anti-inflammatory properties [36,55,56]. Moreover, the identified fatty acid methyl ester (hexadecenoic acid, methyl ester; 9,12-octadecadienoic acid, methyl ester; octadecanoic acid, methyl ester) are known to possess antibacterial, antifungal, and antioxidant activities [57,58].
4. Material and Methods
4.1. Plant Material Sampling and Extract Preparation
Hairy garlic bulbs (Figure 4) were purchased from a local market in 2020 from Hail region (Saudi Arabia). A voucher specimen (AN03) was deposited at the herbarium in the Department of Biology (College of Science, University of Hail, Hail, Kingdom of Saudi Arabia). Briefly, 40 g of bulbs were macerated in 400 mL of distillated pure water at room temperature for 48 h and re-extracted three times using the same procedure. The yield expressed in percentage was calculated using the following equation (Equation (1)):
| Yield (%) = (W1 × 100)/W2 | (1) |
where W1 is the weight of extract after the evaporation of solvent and W2 is the dry weight of the sample.
Figure 4.
Allium subhirsutum L. (bulbs) purchased from a local market in Hail region.
The obtained extract was filtered, and water was lyophilized by using Millirock Technology apparatus (Kingston, NY, USA) to yield amorphous powder. The yield of extraction was about 27.366 ± 0.152%.
4.2. Phytochemical Profiling of Hairy Garlic Aqueous Extract
A Shimadzu Nexis GC-2030 Gas Chromatograph system equipped with a QP2020 NX Mass Spectrometer was used to identify the bioactive compounds in A. subhirsutum L. aqueous extract. Helium was used as a carrier gas in the constant flow mode at 1 mL/min. The initial temperature of the column was 70 °C. It was maintained at this temperature for 2 min and was then gradually increased by 10 °C up to 280 °C. The oven temperature was raised up to 280 °C at the increased rate of 5 °C/min and maintained for 9 min. The injection port temperature was 250 °C and the helium flow rate was 1 mL/min. The ionization voltage was 70 eV. Separation was achieved by a RTSvolatile column about 30 m long. A Quadrupole Mass Detector was employed to detect compounds when they were vented from the column. The temperature of the detector was 300 °C. Using MS data libraries, such as WILEY8.LIB and NIST08, the spectrum was analyzed, and compounds were identified.
The presence of alkaloids, flavonoids, terpenoids, tannins, saponins, steroids, proteins, aminoacids, and cardiac glucosides in hairy garlic aqueous extract was quantified using protocols previously described by Sofowora [59], Trease and Evans [60], and Adetuyi and Popoola [61].
The total phenols content was estimated using the Folin–Ciocalteu method [62]. The total contents of tannins and flavonoids were estimated using the techniques described by Broadhurst and Jones [63] and Benariba et al. [64], respectively.
4.3. Biological Activities
4.3.1. Cytotoxicity Evaluation
The cytotoxicity of the obtained extract was evaluated based on the same protocol carried out by Snoussi et al. [65].
4.3.2. Antimicrobial Activities
The obtained aqueous extract was tested for its ability to act as an antibacterial agent, as previously described by Haddaji et al. [66], against twelve bacteria including four type strains (Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, Proteus mirabilis ATCC 29245, and Klebsiella pneumoniae ATCC 27736) and eight clinical strains (Proteus mirabilis, Staphylococcus sciuri, Streptococcus pyogens, Pseudomonas aeruginosa, multi-drug resistant Staphylococcus aureus, Enterobacter cloacae, Stenotrophomonas paucimobilis, and Acinetobacter baumannii). Three Candida strains (C. albicans ATCC 10231, C. vaginalis, and C. albicans), one C. neoformans ATCC 14116 strain, and two Aspergillus species (A. fumigatus ATCC 204305, A. niger) were also used.
The disk diffusion assay was used for the determination of the diameter of the growth inhibition zone estimated on agar medium. The microdilution assay was used for the determination of the minimal inhibitory concentration (MICs) and the minimal bactericidal/fungicidal concentrations (MBC/MFC). To interpret the mean diameter of the growth inhibition zone obtained on agar media, the scheme proposed by Parveen et al. [67] was used. The results of the (MBC/MIC) and (MFC/MIC) ratios were interpreted using the scheme proposed by Gatsing et al. [68].
Coxsakievirus B-3 (CVB3) and herpes simplex virus type 2 (HSV-2) were used to test the antiviral potential of the aqueous extract using the procedure described by Alreshidi et al. [69].
4.3.3. Antioxidant Activities
The ability of hairy garlic (aqueous extract) to scavenge the DPPH (free-radical 2, 2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals were calculated using the protocol described by Chakraborty and Paulraj [70]. The β-carotene method described by Ikram et al. [71] was used. The ferric-reducing power was estimated following the method described by Bi et al. [72].
4.4. Evaluation of Anti-Quorum Sensing Activity
The inhibition of violacein production was assayed using two starter strains: Chromobacterium violaceum ATCC 14272 and CV026, as previously described by Noumi et al. [73]. On the Lauria–Bertani agar plate, activity was interpreted as moderate when the inhibition zone < 10 mm and potent when zone > 10 mm) [74].
The effect of the A. subhirsutum aqueous extract to inhibit the motility of the Pseudomonas aeruginosa PAO1 strain was tested on 0.3% agar medium (swimming motility) and 0.5% agar medium (swarming motility), as described by Alreshidi et al. [69].
Anti-Biofilm Activity
The antibiofilm activity of hairy garlic aqueous extract was tested against six reference strains obtained from American Type Culture Collection (ATCC, Manassas, Virginia) strains: Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 19433, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Salmonella typhimurium ATCC 14028, and Candida albicans ATCC 10239. The effect 1, 1/2, 1/4, 1/8, and 1/16 MIC concentration value on the biofilm-forming ability of the tested microorganisms was tested using a microplate biofilm assay [75].
The percentage of biofilm inhibition (expressed in percentage) was calculated using the following equation (Equation (2)):
| Inhibition of biofilm formation (%) = [(ODControl − ODSample)/ODControl] × 100 | (2) |
where ODControl is the optical density of the control and ODSample is the optical density of the sample.
4.5. ADMET Profile
The pharmacokinetics and the toxicity profiles of the identified molecules were predicted using a SwissADME online server (http://www.swissadme.ch/, accessed on 19 January 2022) and ProTox-II webserver (http://tox.charite.de/tox/, accessed on 19 January 2022) [76,77,78,79].
4.6. Statistical Analysis
The average values of three replicates were calculated using the SPSS 25.0 statistical package for Windows. Differences in the means were calculated using Duncan’s multiple-range tests for means with a 95% confidence interval (p ≤ 0.05).
5. Conclusions
Overall, we are the first to report the identification of several small peptides and bioactive molecules in the aqueous extract from A. subhirsutum L. using the GC–MS technique. The results obtained allowed the presence of flavonoids (231 ± 0.022 mg QE/g extract), followed by tannins (159 ± 0.006 mg TAE/g extract) and phenols (4 ± 0.004 mg GAE/g extract). Interestingly, several phytocompounds with high biological activities were identified, mainly including 5-hydroxymethylfurfural (37.04%), methyl methanethiolsulfonate (21.33%), furfural (7.64%), beta-D-glucopyranose, 1,6-anhydro- (6.17%), 1,6-anhydro-beta-D-glucofuranose (3.6%), trisulfide, di-2-propenyl (2.70%), and diallyl disulfide (1.93%). These compounds can act independently or synergistically, suggesting their potential application for the treatment of several chronic diseases. Moreover, our results showed significant antioxidant (IC50 values; DPPH 1 mg/mL; ABTS 0.698 ± 0.107 mg/mL; β-carotene 0.811 ± 0.036 mg/mL; and FRAP 1 mg/mL) and antimicrobial properties, as well as antibiofilm properties (up to 62.48 ± 5.50 % against C. albicans ATCC 10239, and 56.21 ± 2.55% against S. aureus ATCC 25923). The tested extract was also able to inhibit the production of violacein by 37.43 ± 0.85% at 5 mg/mL. Swarming and swimming motility in P. aeruginosa PAO1 was inhibited by 23.66 ± 0.5% and 13.67 ± 1%, respectively. Our computational study on the major identified compounds revealed acceptable oral bioavailability of the extract, which can be useful in future bioassay studies. Finally, further study on the isolation of significant molecules in vivo studies is strongly recommended in order to evaluate the effectiveness of the isolates for the desired activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11040495/s1, Supplementary Material S1: Phytoconstituents identified in A. subhirsutum L. aqueous extract using GC-MS technique.
Author Contributions
Conceptualization, M.S. (Mejdi Snoussi), M.A. (Mohd Adnan), M.A. (Mousa Alreshidi), V.D.F. and A.K.; methodology, A.H., L.B., E.N., A.A.-R., M.S. (Mohd Saeed) and O.C.; software, M.A. (Mousa Alreshidi), H.H., A.H. and O.C.; validation, S.G., M.S. (Mejdi Snoussi), A.K., A.A.-R. and K.A.; writing—original draft preparation, A.K.; E.N., M.A. (Mohd Adnan), K.A., M.A. (Mousa Alreshidi), M.S. (Mohd Saeed) and H.H., writing—review and editing, M.S. (Mejdi Snoussi), A.K., L.B., S.G. and E.N.; supervision, M.A. (Mousa Alreshidi), V.D.F., M.S. (Mejdi Snoussi) and A.K.; project administration, M.A. (Mousa Alreshidi) and M.S. (Mejdi Snoussi); funding acquisition, M.A. (Mousa Alreshidi). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scientific Research Deanship at University of Ha’il-Saudi Arabia through project number RG-20141.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated and analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CDC . Antibiotic Resistance Threats in the United States, 2019. Department of Health and Human Services, CDC; Atlanta, GA, USA: 2019. [Google Scholar]
- 2.European Centre for Disease Prevention and Control. [(accessed on 4 November 2020)]. Available online: https://www.ecdc.europa.eu/en/home.
- 3.Alam N., Banu N., Aziz M.A.I., Barua N., Ruman U., Jahan I., Chy F.J., Denath S., Paul A., Chy M.N.U., et al. Chemical Profiling, Pharmacological Insights and In Silico Studies of Methanol Seed Extract of Sterculia foetida. Plants. 2021;10:1135. doi: 10.3390/plants10061135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haque M., Chowdhury A.B.M., Shahjahan M., Harun G.D. Traditional healing practices in rural Bangladesh: A qualitative investigation. BMC Complement. Altern. Med. 2018;18:62. doi: 10.1186/s12906-018-2129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakari S., Hajlaoui H., Daoud A., Mighri H., Ross-Garcia J.M., Gharsallah N., Kadri A. Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of hydroalcoholic extracts of leaves and flowers of Erodium glaucophyllum collected from Tunisian Sahara. Food Sci. Technol. 2018;38:310–317. doi: 10.1590/fst.04517. [DOI] [Google Scholar]
- 6.Mseddi K., Alimi F., Noumi E., Veettil V.N., Deshpande S., Adnan M., Hamdi A., Elkahoui S., Alghamdi A., Kadri A., et al. Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis. Arab. J. Chem. 2020;13:6782–6801. doi: 10.1016/j.arabjc.2020.06.032. [DOI] [Google Scholar]
- 7.Felhi S., Saoudi M., Daoud A., Hajlaoui H., Ncir M., Chaabane R., El Feki A., Gharsallah N., Kadri A. Investigation of phytochemical contents, in vitro antioxidant and antibacterial behavior and in vivo anti-inflammatory potential of Ecballium elaterium methanol fruits extract. Food Sci. Technol. 2017;37:558–563. doi: 10.1590/1678-457x.26316. [DOI] [Google Scholar]
- 8.Felhi S., Hajlaoui H., Ncir M., Bakari S., Ktari N., Saoudi M., Gharsallah N., Kadri A. Nutritional, phytochemical and antioxidant evaluation and FT-IR analysis of freeze-dried extracts of Ecballium elaterium fruit juice from three localities. Food Sci. Technol. 2016;36:646–655. doi: 10.1590/1678-457x.12916. [DOI] [Google Scholar]
- 9.Daoud A., Ben Mefteh F., Mnafgui K., Turki M., Jmal S., Ben Amar R., Ayadi F., ElFeki A., Abid L., Rateb M.E., et al. Cardiopreventive effect of ethanolic extract of date palm pollen against isoproterenol induced myocardial infarction in rats through the inhibition of the angiotensin-converting enzyme. Exp. Toxicol. Pathol. 2017;69:656–665. doi: 10.1016/j.etp.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Alminderej F., Bakari S., Almundarij T.I., Snoussi M., Aouadi K., Kadri. A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants. 2020;9:1534. doi: 10.3390/plants9111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alminderej F., Bakari S., Almundarij T.I., Snoussi M., Aouadi K., Kadri A. Antimicrobial and Wound Healing Potential of a New Chemotype from Piper cubeba L. Essential Oil and In Silico Study on S. aureus tyrosyl-tRNA Synthetase Protein. Plants. 2021;10:205. doi: 10.3390/plants10020205. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Ben Mefteh F., Daoud A., Bouket A.C., Thissera B., Kadri Y., Cherif-Silini H., Eshelli M., Alenezi F.N., Vallat A., Oszako T., et al. Date Palm Trees Root-Derived Endophytes as Fungal Cell Factories for Diverse Bioactive Metabolites. Int. J. Mol. Sci. 2018;19:1986. doi: 10.3390/ijms19071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irfan A., Imran M., Khalid M., Ullah M.S., Khalid N., Assiri M.A., Thomas R., Muthu S., Basra M.A.R., Hussein M., et al. Phenolic and flavonoid contents in Malva sylvestris and exploration of active drugs as antioxidant and anti-COVID19 by quantum chemical and molecular docking studies. J. Saudi Chem. Soc. 2021;25:101277. doi: 10.1016/j.jscs.2021.101277. [DOI] [Google Scholar]
- 14.Zammel N., Saeed M., Bouali N., Elkahoui S., Alam J.M., Rebai T., Kausar M.A., Adnan M., Siddiqui A.J., Badraoui R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods. 2021;10:1383. doi: 10.3390/foods10061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adnan M., Siddiqui A.J., Hamadou W.S., Patel M., Ashraf S.A., Jamal A., Awadelkareem A.M., Sachidanandan M., Snoussi M., De Feo V. Phytochemistry, Bioactivities, Pharmacokinetics and toxicity prediction of Selaginella repanda with its anticancer potential against human lung, breast and colorectal carcinoma cell lines. Molecules. 2021;26:768. doi: 10.3390/molecules26030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badraoui R., Rebai T., Elkahoui S., Alreshidi M., Veettil V.N., Noumi E., Al-Motair A.K., Aouadi K., Kadri A., De Feo V., et al. Allium subhirsutum L. as a potential source of antioxidant and anticancer bioactive molecules: HR-LCMS phytochemical profiling, in vitro and in vivo pharmacological study. Antioxidants. 2020;9:1003. doi: 10.3390/antiox9101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sut S., Maggi F., Bruno S., Badalamenti N., Quassinti L., Bramucci M., Beghelli D., Lupidi G., Dall’Acqua S. Hairy Garlic (Allium subhirsutum) from Sicily (Italy): LC-DAD-MSn analysis of secondary metabolites and in vitro biological properties. Molecules. 2020;25:2837. doi: 10.3390/molecules25122837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emir A., Emir C., Yıldırım H. Characterization of phenolic profile by LC-ESI-MS/MS and enzyme inhibitory activities of two wild edible garlic: Allium nigrum L. and Allium subhirsutum L. J. Food Biochem. 2020;44:e13165. doi: 10.1111/jfbc.13165. [DOI] [PubMed] [Google Scholar]
- 19.Küçük S., Kurkcuoglu M., Tuyan C. Headspace volatiles of Allium subhirsutum L. growing in Turkey. Ann. Phytomed. 2018;7:180–182. doi: 10.21276/ap.2018.7.2.27. [DOI] [Google Scholar]
- 20.Saoudi M., Badraoui R., Chira A., Saeed M., Bouali N., Elkahoui S., Alam J.M., Kallel C., El Feki A. The Role of Allium subhirsutum L. in the attenuation of dermal wounds by modulating oxidative stress and inflammation in Wistar albino rats. Molecules. 2021;26:4875. doi: 10.3390/molecules26164875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nencini C., Menchiari A., Franchi G.G., Micheli L. In vitro antioxidant activity of aged extracts of some Italian Allium species. Plant Foods Hum. Nutr. 2011;66:11–16. doi: 10.1007/s11130-010-0204-2. [DOI] [PubMed] [Google Scholar]
- 22.Loi M., Poli F., Sacchetti G., Selenu M.B., Ballero M. Ethnopharmacology of Ogliastra (Villagrande Strisaili, Sardinia, Italy) Fitoterapia. 2004;75:277–295. doi: 10.1016/j.fitote.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Kunkel G. Plants for Human Consumption: An Annotated Checklist of the Edible Phanerogams and Ferns. Koeltz Scientific Books; Koenigstein, Germany: 1984. [Google Scholar]
- 24.Ertuğ F. Wild edible plants of the Bodrum area (Muǧla, Turkey) Doga Turk. J. Botany. 2004;28:161–174. [Google Scholar]
- 25.Ismail S., Jalilian F.A., Talebpour A.H., Zargar M., Shameli K., Sekawi Z., Jahanshiri F. Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium Boiss. Biomed. Res. Int. 2013;2013:696835. doi: 10.1155/2013/696835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafez Ghoran S., Rahimi H., Kazemi A., Scognamiglio M., Naderian M., Iraji A., Bordbar F. Allium hooshidaryae (Alliaceae); Chemical compositions, biological and ethnomedicine uses. J. Ethnopharmacol. 2021;274:113918. doi: 10.1016/j.jep.2021.113918. [DOI] [PubMed] [Google Scholar]
- 27.El-Saber Batiha G., Magdy Beshbishy A., Wasef L.G., Elewa Y.H., AAl-Sagan A., El-Hack A., Mohamed E., Taha A.E., MAbd-Elhakim Y., Prasad Devkota H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients. 2020;12:872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomšik A., Šarić L., Bertoni S., Protti M., Albertini B., Mercolini L., Passerini N. Encapsulations of wild garlic (Allium ursinum L.) extract using spray congealing technology. Food Res. Int. 2019;119:941–950. doi: 10.1016/j.foodres.2018.10.081. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N., Sun B., Mao X., Chen H., Zhang Y. Flavor formation in frying process of green onion (Allium fistulosum L.) deep-fried oil. Food Res. Int. 2019;121:296–306. doi: 10.1016/j.foodres.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Abe K., Hori Y., Myoda T. Volatile compounds of fresh and processed garlic. Exp. Ther. Med. 2020;19:1585–1593. doi: 10.3892/etm.2019.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M.H., Kim N.H., Heo J.D., Rho J.R., Ock K.J., Shin E.C., Jeong E.J. Comparative evaluation of sulfur compounds contents and antiobesity properties of Allium hookeri prepared by different drying methods. Evid. Based Complement. Altern. Med. 2017;2017:2436927–2436936. doi: 10.1155/2017/2436927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eyupoglu O.E. Antioxidant activities, phenolic contents and electronic nose analysis of black garlic. Int. J. Second. Metab. 2019;6:154–161. doi: 10.21448/ijsm.564813. [DOI] [Google Scholar]
- 33.Takim K., Kutlu T. Determination of phytochemical profile of Allium tuncelianum and evaluation of its antiproliferative effect on various Human cell lines. KSU J. Agric. Nat. 2020;23:259–270. doi: 10.18016/ksutarimdoga.vi.586805. [DOI] [Google Scholar]
- 34.Lu X., Li N., Qiao X., Qiu Z., Liu P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug. Anal. 2017;25:340–349. doi: 10.1016/j.jfda.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C., Cai J., Liu S.Q., Qiu G.L., Wu X.G., Zhang W., Chen C., Qi W.L., Wu Y., Liu Z.B. Comparative study on the composition of four different varieties of garlic. PeerJ. 2019;7:e6442. doi: 10.7717/peerj.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isbilen O., Volkan E. Allium willeanum Holmboe exerts anticancer activities on metastatic breast cancer cells MCF-7 and MDA-MB-231. Heliyon. 2021;7:e07730. doi: 10.1016/j.heliyon.2021.e07730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharazi P.R., Peyvast G. Natural antioxidant and anticarcinogenic compounds from two varieties of Iranian garlic. Asian J. Chem. 2005;17:219–223. [Google Scholar]
- 38.Deryabin D., Galadzhieva A., Kosyan D., Duskaev G. Plant-Derived Inhibitors of AHL-Mediated Quorum Sensing in Bacteria: Modes of Action. Int. J. Mol. Sci. 2019;20:5588. doi: 10.3390/ijms20225588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muzaffer U., Paul V.I. Phytochemical analysis, in vitro antioxidant and antimicrobial activities of male flower of Juglans regia L. Int. J. Food Prop. 2018;21:345–356. doi: 10.1080/10942912.2017.1409762. [DOI] [Google Scholar]
- 40.Al-Haidari R.A., Shaaban M.I., Ibrahim S.R.M., Mohamed G.A. Anti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2016;13:67–71. doi: 10.21010/ajtcam.v13i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohsenipour Z., Hassanshahian M. The Effects of Allium sativum Extracts on Biofilm Formation and Activities of Six Pathogenic Bacteria. Jundishapur J. Microbiol. 2015;8:e18971. doi: 10.5812/jjm.18971v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatwalkar S.B., Gound S.S., Mondal R., Srivastava R.K., Anupam R. Anti-biofilm and Antibacterial Activity of Allium sativum Against Drug Resistant Shiga-Toxin Producing Escherichia coli (STEC) Isolates from Patient Samples and Food Sources. Indian J. Microbiol. 2019;59:171–179. doi: 10.1007/s12088-019-00784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caputo L., Amato G., Fratianni F., Coppola R., Candido V., De Feo V., Nazzaro F. Chemical Characterization and Antibiofilm Activities of Bulbs and Leaves of Two Aglione (Allium ampeloprasum var. holmense Asch. et Graebn.) Landraces Grown in Southern Italy. Molecules. 2020;25:5486. doi: 10.3390/molecules25235486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L., Chen J., Su J., Li L., Hu S., Li B., Zhang X., Xu Z., Chen T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food. Chem. 2013;61:10604–10611. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
- 45.Li M.M., Wu L.Y., Zhao T., Xiong L., Huang X., Liu Z.H., Fan X.L., Xiao C.R., Gao Y., Ma Y.B., et al. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones. 2011;16:267–273. doi: 10.1007/s12192-010-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding X., Wang M.Y., Yu Z.L., Hu W., Cai B.C. Studies on separation, appraisal and the biological activity of 5-HMF in Cornus officinalis. Zhongguo Zhong Yao Za Zhi. 2008;33:392–396. [PubMed] [Google Scholar]
- 47.Joller C., De Vrieze M., Moradi A., Fournier C., Chinchilla D., L’Haridon F., Bruisson S., Weisskopf L. S-methyl methanethiosulfonate: Promising late blight inhibitor or broad range toxin? Pathogens. 2020;9:496. doi: 10.3390/pathogens9060496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy B.S., Kawamori T., Lubet R., Steele V., Kelloff G., Rao C.V. Chemopreventive effect of S-methylmethane thiosulfonate and sulindac administered together during the promotion/progression stages of colon carcinogenesis. Carcinogenesis. 1999;20:1645–1648. doi: 10.1093/carcin/20.8.1645. [DOI] [PubMed] [Google Scholar]
- 49.Vijayakumar K., Ramanathan T. Antiquorum sensing and biofilm potential of 5-Hydroxymethylfurfural against Gram positive pathogens. Microb. Pathog. 2018;125:48–50. doi: 10.1016/j.micpath.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Chai W.-M., Liu X., Hu Y.-H., Feng H.-L., Jia Y.-L., Guo Y.-J., Zhou H.T., Chen Q.-X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013;57:151–155. doi: 10.1016/j.ijbiomac.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Rao P.S., Midde N.M., Miller D.D., Chauhan S., Kumar A., Kumar S. Diallyl sulfide: Potential use in novel therapeutic interventions in alcohol, drugs, and disease mediated cellular toxicity by targeting cytochrome P450 2E1. Curr. Drug Metab. 2015;16:486–503. doi: 10.2174/1389200216666150812123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song X., Yue Z., Nie L., Zhao P., Zhu K., Wang Q. Biological functions of diallyl disulfide, a garlic-derived natural organic sulfur compound. Evid. Based Complement. Alternat. Med. 2021;2021:5103626. doi: 10.1155/2021/5103626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puccinelli M.T., Stan S.D. Dietary bioactive diallyl trisulfide in cancer prevention and treatment. Int. J. Mol. Sci. 2017;18:1645. doi: 10.3390/ijms18081645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukao T., Hosono T., Misawa S., Seki T., Ariga T. Chemoprotective effect of diallyl trisulfide from garlic against carbon tetrachloride-induced acute liver injury of rats. Biofactors. 2004;21:171–174. doi: 10.1002/biof.552210135. [DOI] [PubMed] [Google Scholar]
- 55.Win D.T. Oleic acid—The anti-breast cancer component in olive oil. AU J.T. 2005;9:75–78. [Google Scholar]
- 56.Harada H., Yamashita U., Kurihara H., Fukushi E., Kawabata J., Kamei Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002;22:2587–2590. [PubMed] [Google Scholar]
- 57.Pinto M., Araújo S., Morais M., Sá N., Lima C., Rosa C., Siqueira E., Johann S., Lima L. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. Anais da Academia Brasileira de Ciências. 2017;89:1671–1681. doi: 10.1590/0001-3765201720160908. [DOI] [PubMed] [Google Scholar]
- 58.Rahman M.M., Ahmad S.H., Mohamed M.T.M., Ab Rahman M.Z. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Sci. World J. 2014;2014:635240. doi: 10.1155/2014/635240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sofowora A. Medicinal Plants and Traditional Medicine in West Africa. John Wiley and Sons; New York, NY, USA: 1982. [Google Scholar]
- 60.Trease G.E., Evans W.C. Pharmacognosy. 11th ed. Cassell and Collier Macmillan Publishers Ltd.; London, UK: 1978. 784p [Google Scholar]
- 61.Adetuyi A.O., Popoola A.V. Extraction and dyes ability potential studies of the colourant in Zanthoxylum zanthoxyloides plant on cotton fabric. J. Sci. Eng. Technol. 2001;8:3291–3299. [Google Scholar]
- 62.Kumar M., Gupta V., Kumari P., Reddy C., Jha B. Assessment of nutrient composition and antioxidant potential of Caulerpaceae seaweeds. J. Food Compos. Anal. 2011;24:270–278. doi: 10.1016/j.jfca.2010.07.007. [DOI] [Google Scholar]
- 63.Broadhurst R.B., Jones W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978;29:788–794. doi: 10.1002/jsfa.2740290908. [DOI] [Google Scholar]
- 64.Benariba N., Djaziri R., Bellakhdar W., Belkacem N., Kadiata M., Malaisse W. Phytochemical screening and free radical scavenging activity of Citrullus colocynthis seeds extract. Asian Pac. J. Trop. Biomed. 2013;3:35–40. doi: 10.1016/S2221-1691(13)60020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snoussi M., Trabelsi N., Dehmeni A., Benzekri R., Bouslama L. Phytochemical analysis, antimicrobial and antioxidant activities of Allium roseum var. odoratissimum (Desf.) Coss extracts. Ind. Crop. Prod. 2016;89:533–542. doi: 10.1016/j.indcrop.2016.05.048. [DOI] [Google Scholar]
- 66.Haddaji F., Papetti A., Noumi E., Colombo R., Deshpande S., Aouadi K., Adnan M., Kadri A., Selmi B., Snoussi M. Bioactivities and in silico study of Pergularia tomentosa L. phytochemicals as potent antimicrobial agents targeting type IIA topoisomerase, TyrRS, and Sap1 virulence proteins. Environ. Sci. Pollut. Res. Int. 2021;28:25349–25367. doi: 10.1007/s11356-020-11946-y. [DOI] [PubMed] [Google Scholar]
- 67.Parveen M., Ghalib R.M., Khanam Z., Mehdi S.H., Ali M. A novel antimicrobial agent from the leaves of Peltophorum vogelianum (Benth.) Nat. Prod. Res. 2010;24:1268–1273. doi: 10.1080/14786410903387688. [DOI] [PubMed] [Google Scholar]
- 68.Gatsing D., Tchakoute V., Ngamga D., Kuiate J.-R., Tamokou J.D.D., Nji-Nkah B.F., Tchouanguep F.M., Fodouop S.P.C. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009;34:126–136. [Google Scholar]
- 69.Alreshidi M., Noumi E., Bouslama L., Ceylan O., Veettil V.N., Adnan M., Danciu C., Elkahoui S., Badraoui R., Al-Motair K.A., et al. Phytochemical Screening, Antibacterial, Antifungal, Antiviral, Cytotoxic, and Anti-Quorum-Sensing Properties of Teucrium polium L. Aerial Parts Methanolic Extract. Plants. 2020;9:1418. doi: 10.3390/plants9111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chakraborty K., Paulraj R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010;122:31–41. doi: 10.1016/j.foodchem.2010.02.012. [DOI] [Google Scholar]
- 71.Ikram E.H.K., Eng K.H., Jalil A.M.M., Ismail A., Idris S., Azlan A., Nazri H.S.M., Diton N.A.M., Mokhtar R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal. 2009;22:388–393. doi: 10.1016/j.jfca.2009.04.001. [DOI] [Google Scholar]
- 72.Bi H., Gao T., Li Z., Ji L., Yang W., Jeff Iteku B., Liu E., Zhou Y. Structural elucidation and antioxidant activity of a water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries) Food Chem. 2013;138:1470–1475. doi: 10.1016/j.foodchem.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 73.Noumi E., Merghni A., Alreshidi M.M., Haddad O., Akmadar G., De Martino L., Mastouri M., Ceylan O., Snoussi M., Al-sieni A., et al. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules. 2018;23:2672. doi: 10.3390/molecules23102672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaki A.A., Shaaban M.I., Hashish N.E., Amer M.A., Lahloub M.F. Assessment of anti-quorum sensing activity for some ornamental and medicinal plants native to Egypt. Sci. Pharm. 2013;81:251–258. doi: 10.3797/scipharm.1204-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ceylan O., Tamfu A.N., Doğaç Y.İ., Teke M. Antibiofilm and anti-quorum sensing activities of polyethylene imine coated magnetite and nickel ferrite nanoparticles. 3 Biotech. 2020;10:513. doi: 10.1007/s13205-020-02509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kadri A., Aouadi K. In vitro antimicrobial and α-glucosidase inhibitory potential of enantiopure cycloalkylglycine derivatives: Insights into their in silico pharmacokinetic, druglikeness, and medicinal chemistry properties. J. App. Pharm. Sci. 2020;10:107–115. [Google Scholar]
- 77.Othman I.M.M., Gad-Elkareem M.A.M., Anouar E.H., Aouadi K., Kadri A., Snoussi M. Design, synthesis ADMET and molecular docking of new imidazo[4,5-b]pyridine-5-thione derivatives as potential tyrosyl-tRNA synthetase inhibitors. Bioorg. Chem. 2020;102:104105. doi: 10.1016/j.bioorg.2020.104105. [DOI] [PubMed] [Google Scholar]
- 78.Ghannay S., Kadri A., Aouadi K. Synthesis, in vitro antimicrobial assessment, and computational investigation of pharmacokinetic and bioactivity properties of novel trifluoromethylated compounds using in silico ADME and toxicity prediction tools. Monatshefte für Chemie. 2020;151:267–280. doi: 10.1007/s00706-020-02550-4. [DOI] [Google Scholar]
- 79.Othman I.M., Gad-Elkareem M.A., Snoussi M., Aouadi K., Kadri A. Novel fused pyridine derivatives containing pyrimidine moiety as prospective tyrosyl-tRNA synthetase inhibitors: Design, synthesis, pharmacokinetics and molecular docking studies. J. Mol. Struct. 2020;1219:128651. doi: 10.1016/j.molstruc.2020.128651. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated and analyzed during this study are included in this article.