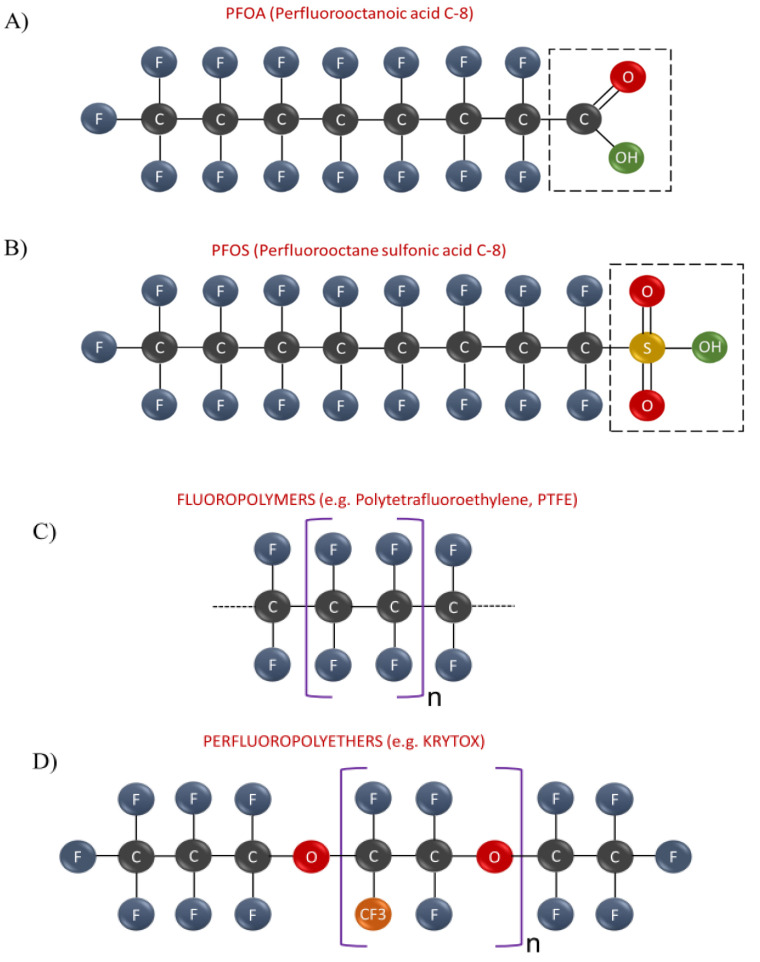
Figure 2.
Examples of non-polymeric and polymeric PFAS substances. (A,B) PFOA and PFOS, two well-known non-polymeric PFAS are shown. Both these compounds possess a relatively long tail containing eight fluorinated carbon atoms, but differ in the chemical composition of the polar head group, which is a carboxylic acid for PFOA and a sulfonic acid for PFOS. Under specific pH environmental conditions, these functional groups can dissociate into the respective anion forms (i.e., carboxylate and sulfonate). (C) Exemplary structure of Polytetrafluoroethylene (PTFE), also known as Teflon, a polymeric PFAS belonging to the subgroup of fluoropolymers. This type of compound is constituted by a moiety of (CF2-CF2)n atoms which is repeated up to thousands of times; (D) Exemplary structure of a lubrificant also known as Krytox, which is a polymeric PFAS belonging to the subgroup of perfluoropolyethers. In this case the (CF[CF3]−CF2−O)n moiety is repeated between 10–60 times.