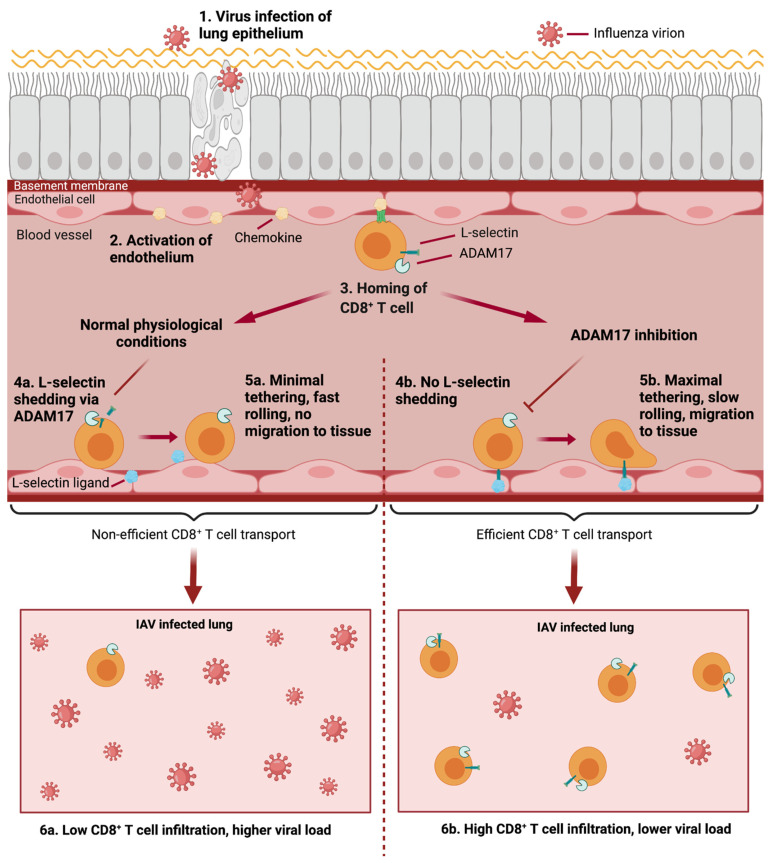
Figure 5.
The role of ADAM17-mediated control of L-selectin in CD8+ T cell homing in IAV infection. 1. Influenza virions infect airway epithelium. 2. Epithelial infection results in activation of blood vessel endothelial cells and expression of chemokines. 3. During homing, CD8+ T cells become activated by chemokines, stimulating A disintegrin and metalloproteinase 17 (ADAM17). This leads to two possible outcomes depending on the level of ADAM17-dependent shedding of L-selectin (CD62L). Under normal conditions: 4a. L-selectin will become proteolytically cleaved by ADAM17. 5a. Loss of L-selectin ectodomain results in minimal CD8+ T cell tethering and rolling along endothelium. T cells fail to migrate to influenza A virus (IAV) infected tissue. 6a. IAV infected lungs will contain lower CD8+ T cell infiltration and will therefore result in higher viral load. When ADAM17 dependent L-selectin shedding is inhibited: 4b. Blocking ADAM17 function retains L-selectin expression on the surface of CD8+ T cells. 5b. L-selectin binding to its ligand allows maximal CD8+ T cell tethering and rolling along endothelium. T cells migrate to influenza A virus (IAV) infected tissue. 6b. IAV infected lungs will contain higher CD8+ T cell infiltration and will therefore result in lower viral load [2].