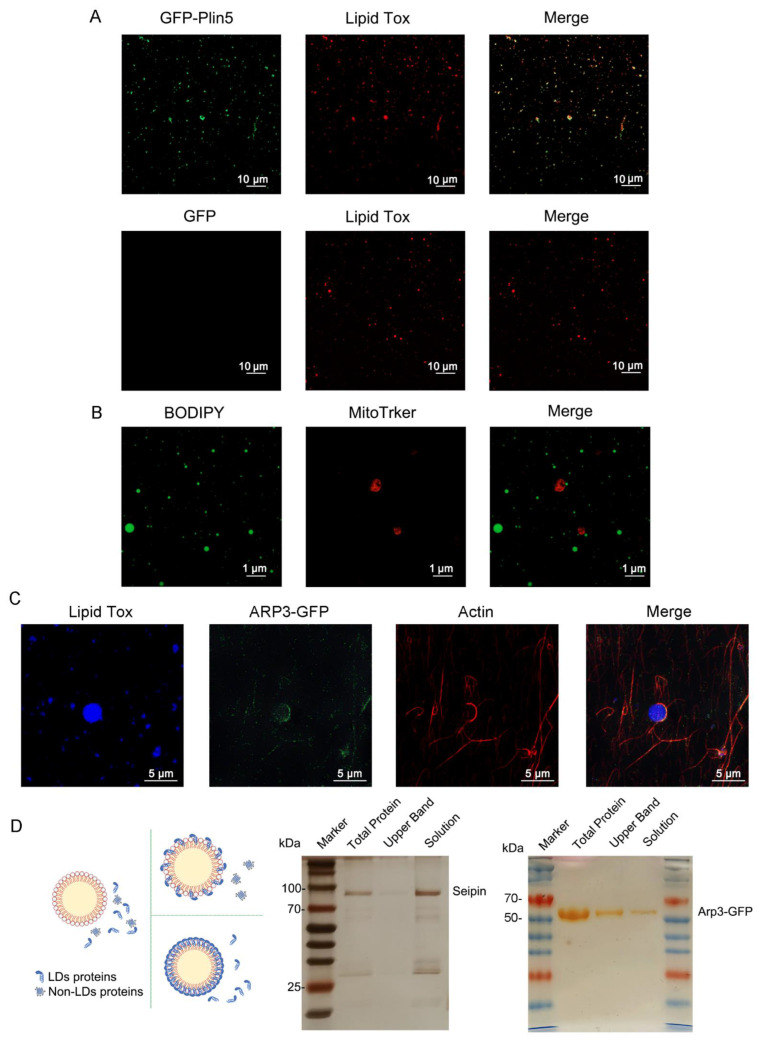
Figure 3.
Organelles and specific protein recruitment of NLPs. (A) Plin5-GFP fusion proteins could be recruited to aLDs. Ten micrograms of GFP and Plin5-GFP fusion proteins were incubated with NLPs for 2 h. Then, the aLDs were stained with Lipid Tox and observed by confocal microscopy. Scale bars, 10 µm. (B) NLPs could be recruited to mitochondria. Extracted mitochondria were incubated with NLPs for 1 h. ALDs and mitochondria were stained with BODIPY493/503 and MitoTracker Red CMXRos, respectively, and subsequently observed by confocal microscopy. Scale bars, 1 µm. (C) NLPs could be recruited to microfilaments and surrounded by microfilaments in the shape of “hooks”. ARP3-GFP colocalized with microfilaments and was enriched at the contact site of all three proteins. NLPs, ARP3-GFP fusion protein and microfilaments were coincubated for 1 h. NLPs and microfilaments were stained with LipidTox and phalloidin, respectively, and subsequently observed by confocal microscopy. Scale bars, 5 µm. (D) Seipin was not recruited to NLPs. Ten micrograms of seipin were incubated with NLPs for 2 h. Arp3 was recruited to NLPs. Ten micrograms of Arp3 were incubated with NLPs for 2 h. The results were analyzed using silver staining. Only lipid droplet-resident/structure-like proteins were recruited to NLPs. Other types of proteins, such as multiple transmembrane proteins, were not recruited to NLPs.