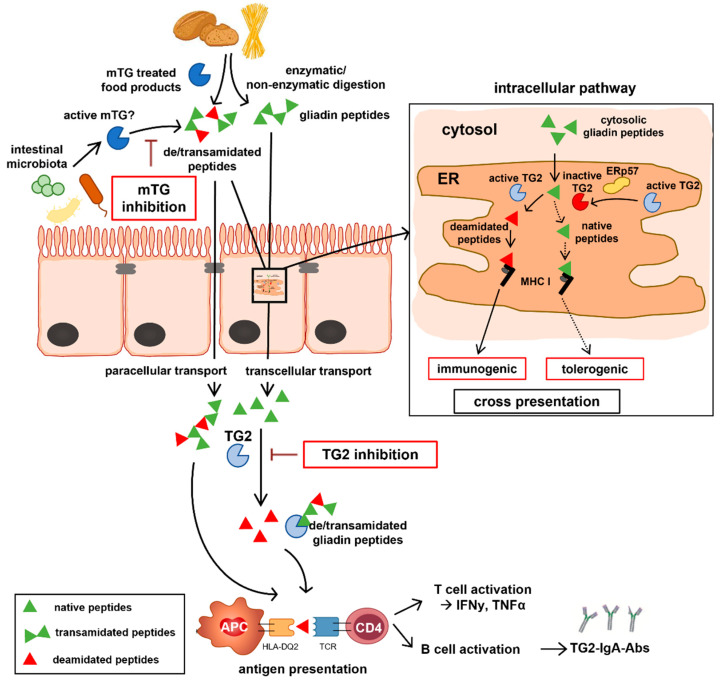
Figure 5.
Role of intra/extracellular TG2 and mTG within CD pathogenesis. Gliadin peptides are resistant to enzymatic and non-enzymatic digestion and reach the intestinal lamina propria by trans- and paracellular routes. Enzymatic modification of gliadin peptides by TG2 increases their antigenicity and is a prerequisite for MHC class II mediated antigen presentation resulting in T and B cell activation. mTG used in food production can de- and/or transamidate gliadin peptides and create neo-epitopes, whereas active mTG released from the intestinal microbiota could modify peptides within the gut lumen. At the intracellular pathway, gliadin peptides reach the ER by phagosome-to-cytosol pathway. Within the ER, deamidation of gliadin peptides by active TG2 and mTG might occur, an effect that could be reduced via inhibition of TG2 (but not of mTG) by ERp57. The corresponding deamidated peptides might serve as substrate for MHC class I mediated immunogenic cross presentation ending up by the action of intraepithelial lymphocytes in villous atrophy, whereas native peptides could potentially induce tolerance via cross presentation.