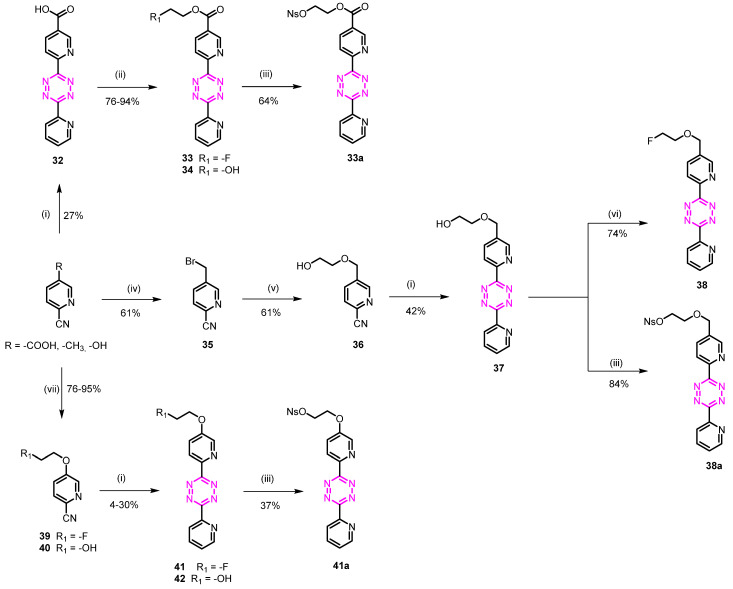
Scheme 4.
Reagents and conditions: (i) 2-pyridinecarbonitrile, (a) NH2NH2.H2O, S8, EtOH, 90 °C, 4 h; (b) NaNO2, AcOH, 0 °C to rt, 30 min; (ii) 1-fluoro-2-iodoethane or 2-bromoethanol, DIPEA, DMF, 70 °C, 4 h; (iii) nosyl chloride, DIPEA, DMAP, CH2Cl2, rt, 1 h; (iv) NBS, AIBN, CH3CN, reflux, 12 h; (v) ethylene glycol, NaH, THF, 0 °C to reflux, 12 h; (vi) CF3(CF2)3SO2F, Et3N.3HF, THF, rt, 18 h; (vii) 1-fluoro-2-iodoethane or 2-bromoethanol, K2CO3, CH3CN, 70 °C, 12 h.