Table 1.
18F-labeling of tetrazines developed in this study.
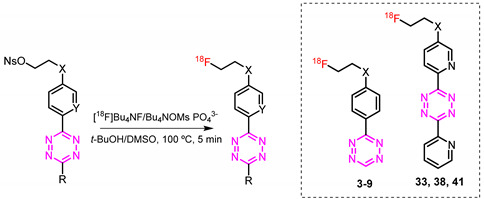
| ||||||
|---|---|---|---|---|---|---|
| Tetrazine | X | R | RCC (%) * Low Precursor Amount (3.1 nmol) |
RCC (%) * High Precursor Amount (9.3 nmol) |
||
| HPLC | TLC | HPLC | TLC | |||
| 3 | -COO- | -H | 0 | 0 | 18 ± 8 | 16 ± 6 |
| 5 | -CH2COO- | -H | - b | - b | - b | - b |
| 6 | -CH2CONH- | -H | - b | - b | - b | - b |
| 7 | -O- | -H | 45 ± 6 | 52 ± 4 | 53 ± 5 | 55 ± 4 |
| 8 | -CH2O- | -H | 23 ± 4 | 24 ± 3 | 26 ± 4 | 28 ± 3 |
| 9 c | -CH2NH- | -H | - d | - d | - d | - d |
| 33 | -COO- | -2-Pyr | 24 ± 3 | 22 ± 3 | 27 ± 4 | 25 ± 5 |
| 38 | -O- | -2-Pyr | 20 ± 11 | 21 ± 8 | 29 ± 10 | 28 ± 6 |
| 41 | -CH2O- | -2-Pyr | 9 ± 1 | 8 ± 1 | 23 ± 3 | 26 ± 6 |
a Precursor was not obtained. b No reaction observed. c Precursor protected with trityl. d Precursor decompose during reaction. n.d. = not determined. * Radiochemical conversions (RCC) were calculated for each compound by radio-HPLC and radio-TLCs as recently reported [43]. All results were based on n = 3 experiments.
