Table 2.
Structural scaffolds, calculated physicochemical properties (TPSA, clogD7.4), and blocking efficiencies of all investigated tetrazine derivatives. Compound 45 displayed a suitable profile to be translated into preclinical studies.
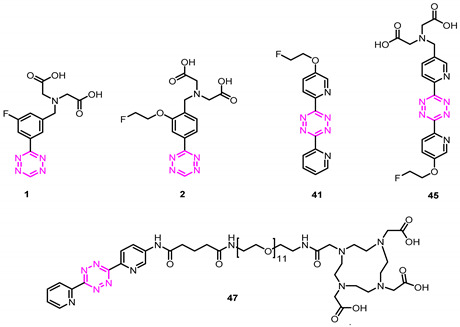
| ||||
|---|---|---|---|---|
| Tetrazine | clogD7.4 b | TPSA c | Blocking Effect f | % Tumor Uptake of [111In]47 after Blocking |
| 1 a | −6.93 | 129 | 90 ± 5 g | 10 ± 5 g |
| 2 a | −6.83 | 138 | 98 ± 0.3 h | 2 ± 0.3 h |
| 41 * | 3.65 | 35 | 22 ± 21 | 78 ± 21 |
| 45 a | −6.36 | 164 | 99 ± 0.7 | 1 ± 0.7 |
| 47 d | −4.13 e | 358 | 99 ± 0.5 | 1 ± 0.5 |
a The compounds were obtained as trifluoroacetate salt. b Calculated distribution coefficient at physiological pH (7.4) using Chemicalize software. c Calculated using Chemicalize software. d The compound was employed as a reference. e Calculated as chelated to a trivalent cation. f The blocking effect of nonradiolabeled Tz was determined as the change in tumor uptake of [111In]47 22 h p.i. Each Tz was administered 1 h prior to [111In]47, and the uptake was normalized to a group of animals in which no blocking was performed (control). Data represent mean from n = 3 mice/group; detailed information can be found in the Materials and Methods section. g Blocking data from [26]. h Blocking data from [27]. * Tz 41 was tested to demonstrate that the blocking effect was related to clogD7.4 and not the bispyridyl scaffold. TPSA = topological polar surface area.
