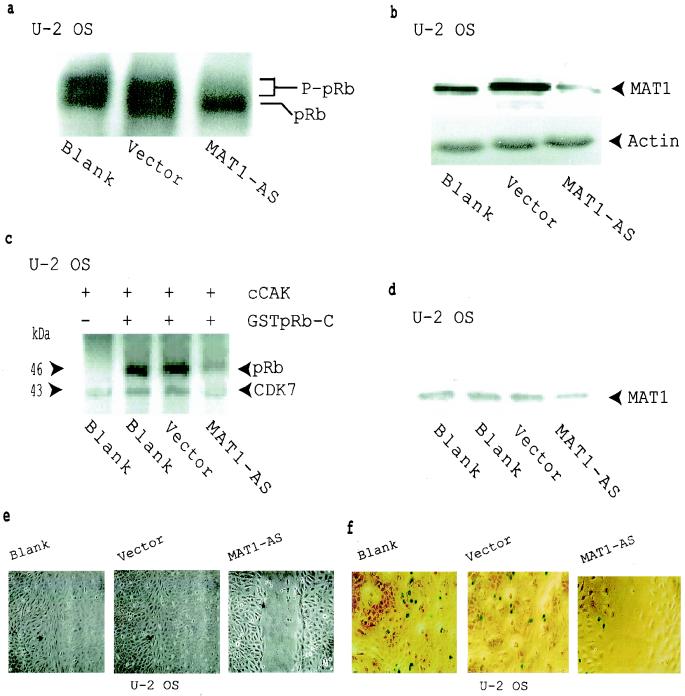
FIG. 2.
Inhibition of pRb phosphorylation and cell proliferation in MAT1-AS-transduced U-2 OS cells. (a) Western blotting shows that pRb phosphorylation was inhibited in MAT1-AS-transduced cells. P-pRB, hyperphosphorylated form of pRB. (b) Western blotting analysis of MAT1 expression. Actin detection was performed on the same blot as the protein loading control. (c) cCAK phosphorylation of pRb. cCAKs immunoprecipitated from transduced and nontransduced U-2-OS cells were used as enzymes for the kinase assay; GSTpRb-C was used as a substrate. CDK7 was autophosphorylated in these reactions. (d) Equal amounts of CAK immunoprecipitates used in panel c were resolved by SDS-PAGE for Western blotting detection of the MAT1 level. (e) Analyses of cell proliferation activation in U-2 OS cells as described in the legend to Fig. 1c. The time for closure of the wound track in vector-transduced and nontransduced blank cells was 72 h, while the wound track in MAT1-AS-transduced cells was still open at that time. The time for closure of the wound track in MAT1-AS-transduced cells was 120 h (data not shown). (f) Cell proliferation was tested by retrovirus-mediated β-galactosidase transduction. Transduced and nontransduced confluent U-2 OS cells were scraped to release cells from contact inhibition. At 24 h after the wound track was created, proliferation activation was tested by transduction of the retroviral G1nBgSvNa vector into the wound track. At 48 h posttransduction, activated U-2 OS cell proliferation was measured at the margin of the wound track by counting the blue β-galactosidase-positive cells upon exposure to X-Gal under a phase-contrast microscope. The percentage of β-galactosidase-positive cells was 9% in MAT1-AS-transduced cells, 32% in nontransduced blank cells, and 30% in vector-transduced cells.