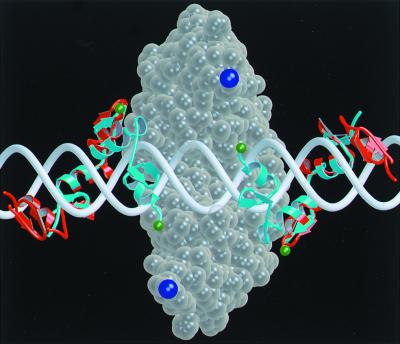
FIG. 4.
Molecular model of the domains of the chimeric nuclease on DNA. The cleavage domain dimer (ball representation in transparent wheat) sits largely behind the DNA (white) in this view and reaches around the duplex at the top and bottom. The zinc finger domains wind through the major groove and are shown in ribbon representation centered around the cleavage dimer: cyan for binding sites separated by 6 bp and red for a 10-bp separation. The residues that must be connected by the flexible linker are colored green on the zinc finger domains and dark blue on the cleavage domains. In moving from the 6-bp to the 10-bp separation, the attachment sites for the linker have retracted both axially and into the plane of the picture. The distance that the linker must extend to join the binding and cleavage domains has gone from about 20 Å for the 6-bp spacer to >30 Å for the 10-bp case. If the linker cannot reach this distance once the zinc finger domains are bound, the cleavage domain cannot dimerize. This model (52) was produced with the program O, and the figure was generated with MolScript.