FIG. 10.
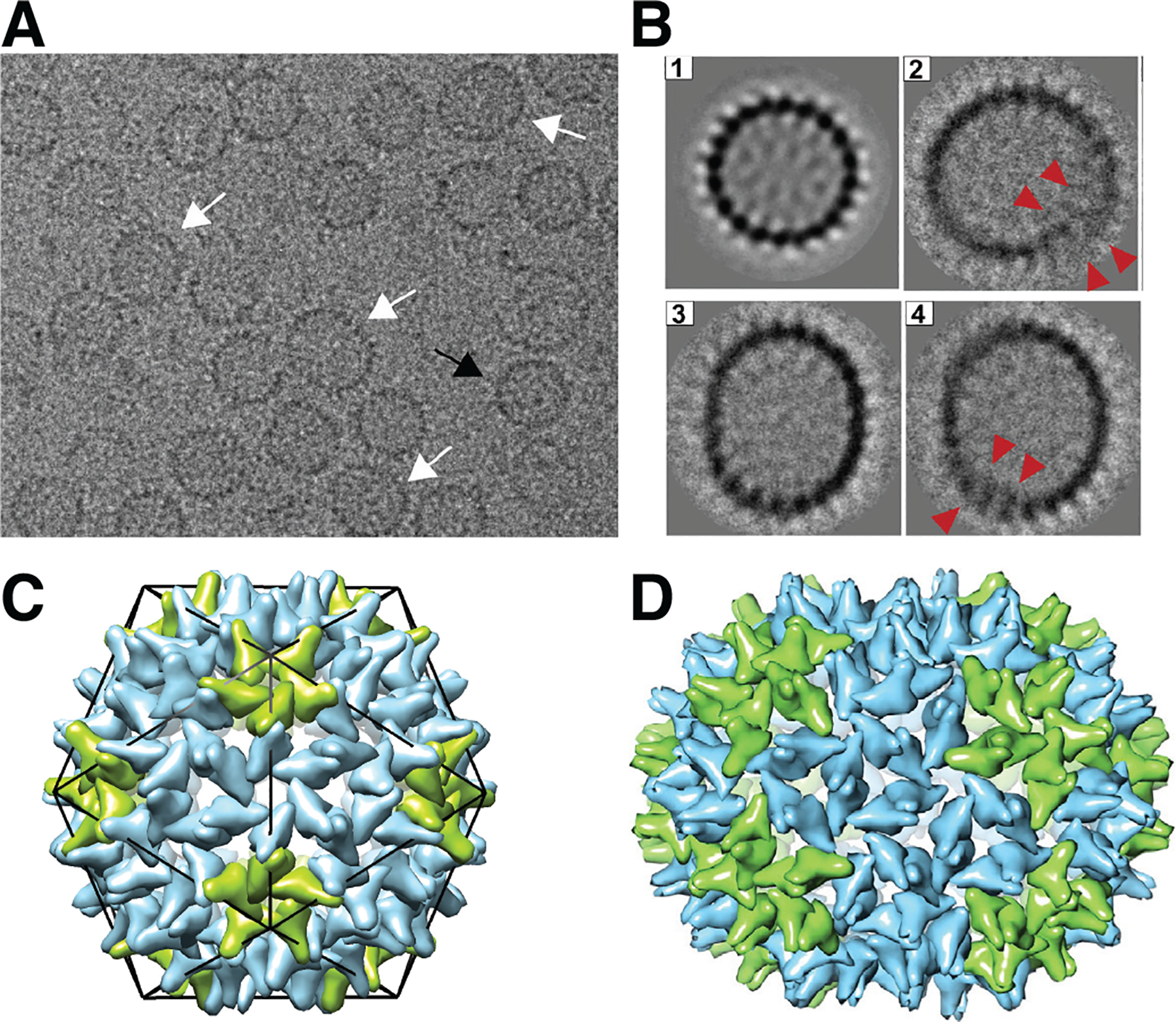
Examples of well-formed and defective woodchuck hepatitis B virus (WHV) capsids, adapted from Ref. (Pierson et al., 2016). (A) Portion of a representative cryo-EM image of empty WHV capsids assembled in vitro in the absence of RNA (from capsid proteins with the C-terminal RNA binding deleted, wCp149). The population of capsids is structurally heterogeneous; the black arrow indicates an example of a T = 4 icosahedral capsid, while the white arrows indicate examples of defective capsids. (B) 2D class averages of (1) icosahedral and (2–4) defective particles. Red arrows in (2) and (4) indicate locations where the capsid shell has overgrown and overlaps itself. (C) Schematic model of a T = 4 icosahedral HBV capsid, with the monomers forming the 12 five-fold vertices colored green, and others colored blue. (D) Hypothetical model of the structure of a capsid that is non-icosahedral but closed, with an elongated structure containing 150 protein dimers (the icosahedral capsid has 120 dimers). Here the green dimers are in pentamers or extend between pentamers and hexamers, and the blue dimers are in hexamers or extend between two hexamers. Images reprinted from (Pierson et al., 2016).
