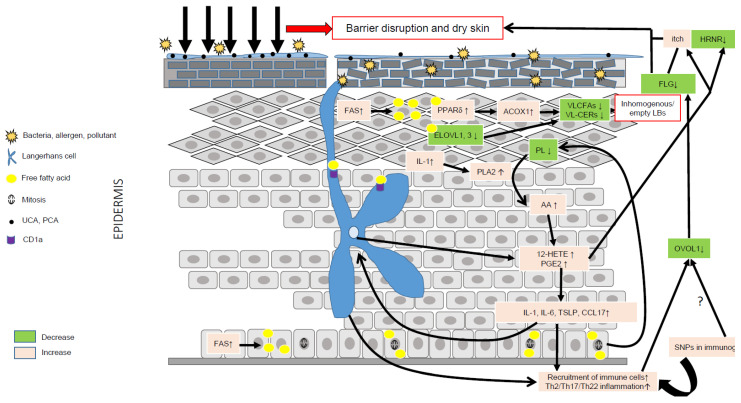
Figure 3.
Role of lipid metabolism in AD pathogenesis. Environmental factors such as the microbiota, pollution, climate, allergens and stress can significantly alter the epidermal barrier and lead to dry skin. Compensatory mechanisms aimed at repairing the barrier include production of pro-inflammatory cytokines such as IL-1, up-regulation of FFA synthesis via FAS, increased synthesis of CHOL via HMGCoA reductase, and of proteins and DNA. IL-1 is a pleiotropic cytokine able to stimulate the hydrolysis of membrane phospholipids via PLA2 and the liberation of AA. AA is then metabolized into eicosanoids, such as 12-HETE and PGE2, together able to evoke itch, promote inflammation and reduce the expression of hornerin (HRNR), hence contributing to further impairment of the epidermal barrier. Increased synthesis of FFAs can directly activate the transcriptional activity of PPARδ, which, in turn, increases the metabolism of VLCFAs via up-regulation of ACOX1. The latter, combined with a reduction of ELOVL1 and -3, leads to reduced levels of VLC-CERs and VLC-FFAs in the epidermis, hence modifying the composition of the SC lipid matrix. VLC-CERs and VLC-FFAs are used as energetic substrates to sustain KC hyperproliferation rather than as structural lipids, hence leading to the synthesis of empty or inhomogeneous lamellar bodies (LBs). Abnormal lipid metabolism resulting in fewer antimicrobial lipids might blunt the innate immune response, hence favoring skin superinfection. Of note, the immature skin of young children contains lower amounts of antimicrobial lipids. LCs can produce 12-HETE, thereby significantly contributing to the local pro-inflammatory milieu, and, via CD1a, take up lipid neoantigens produced in activated KCs or secreted by bacteria. LCs can also sense changes in the skin microbiota, and take up allergens or directly be activated by pro-inflammatory cytokines such as TSLP. Then, activated LCs migrate to skin draining lymph nodes where they prime Th2 T cells. Th2 cytokines, such as IL-13, can down-regulate the OVOL1 pathway, potentially similar to SNPs, leading to dampened FLG synthesis and, in turn, contributing to reduced skin moisture. The secretion of abnormal LBs and the diminution of FLG associated with genetic variants (SNPs) in immunogenic genes might create a vicious cycle perpetuating AD.