Abstract
Intraventricular hemorrhage (IVH) remains a major complication of prematurity, worldwide. The severity of IVH is variable, ranging from a tiny germinal matrix bleed to a moderate-to-large ventricular hemorrhage or periventricular hemorrhagic infarction. Survivors with IVH often suffer from hydrocephalus and white matter injury. There is no tangible treatment to prevent post-hemorrhagic cerebral palsy, cognitive deficits, or hydrocephalus in these infants. White matter injury is attributed to blood-induced damage to axons and maturing oligodendrocyte precursors, resulting in reduced myelination and axonal loss. Hydrocephalus results from obstructed CSF circulation by blood clots, increased CSF production, and reduced CSF absorption by lymphatics and arachnoid villi. Several strategies to promote neurological recovery have shown promise in animal models, including elimination of blood and blood products, alleviating cerebral inflammation and oxidative stress, as well as promoting survival and maturation of oligodendrocyte precursors. The present review integrates novel mechanisms of brain injury in IVH and the imminent therapies to alleviate post-hemorrhagic white matter injury and hydrocephalus in the survivors with IVH.
Keywords: intraventricular hemorrhage, white matter injury, post-hemorrhagic hydrocephalus, inflammation, oxidative stress, oligodendrocyte progenitor cells, stem cells
INTRODUCTION
Germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) remains a major problem of extremely premature infants worldwide, affecting about 20% of very low birth weight infants (<1500g) each year in the USA.1 IVH spontaneously develops in extremely preterm infants admitted in the neonatal intensive care units usually within the first three days of life. As these infants recover and grow older, they often suffer from neurologic sequelae, including hydrocephalus, cerebral palsy (CP), and cognitive deficits. With advancement in technology and care over the last few decades, the survival of these infants has improved and a paradigm shift in the comorbidities has emerged. 2 The cases of mild CP and minor cognitive deficits have increased, while severe CP and major developmental impairments are becoming less common.
IVH commonly occurs in premature infants of less than 32 weeks of gestational age. The hemorrhage initiates usually in the germinal matrix and extends into the cerebral ventricle with rupture of the underlying ependyma. The subpendymal germinal matrix consists of neuronal and glial precursor cells. Indeed, GMH-IVH affects survival, production and maturation of these progenitors. IVH was classified by Lu-Ann Papile (1978) into 4 grades that still remains the most prevalent classification of IVH.3 Grade 1, hemorrhage confined to the germinal matrix; Grade 2, IVH without ventricular dilation; Grade 3, IVH with ventricular dilation; Grade IV, IVH with intraparenchymal hemorrhage. Intra-parenchymal echodensities regarded as grade IV IVH are often periventricular hemorrhagic infarction, and not an extension of IVH. Most of these infants remain asymptomatic and diagnosis is made by a screening head ultrasound. Some patients exhibit minor abnormalities in consciousness, movement, tone, respiration, and eye movement. Follow up head ultrasounds are performed in infants with grade II-IV IVH to assess the progression in ventriculomegaly and periventricular injury. In addition, head ultrasound or magnetic resonance imaging (MRI) is obtained in all these infants at near term (35-42 weeks) to detect injury to the periventricular white matter and other brain regions, which predicts their long-term developmental outcome. As these infants are at high risk to developing neurological and other health problems, they are followed in Neonatal Follow Up Clinics and by Early Intervention Program (EIP) after discharge from Neonatal Intensive Care Units (NICU). EIPs are publicly funded service for disabled children in the USA, whereas European Agency for Special Needs and Inclusive Education offers care to these children in Europe.
The infants with grade III-IV IVH have a survival rate of 40-45%, but more than 80% of infants with grade I-II hemorrhage survive.4, 5 In a large study conducted in 16 centers of National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN; n=2514), about 9% of Infants with grade I-II IVH and 28% of Infants with grade III-IV IVH develop CP compared to 8% of Infants without IVH.6 Moreover, the cognitive and language scores were worse In neonates with grade III-VI IVH compared with grade I-II. Indeed, The risk of neurodevelopmental Impairment Is much higher In grade III-IV IVH cases, and a half to two-thirds of these Infants develop CP, cognitive deficit, Intellectual disability and/or hydrocephalus.7-9 Together, the occurrence of IVH takes a huge toll emotionally, physically, and financially on the families and society In general. The treatment of neurodevelopmental Impairment Is limited to physical and occupational therapy; the Infants with hydrocephalus often undergo placement of reservoirs and shunt surgery that requires frequent revisions.
Pathology
The common pathological findings Include germinal matrix destruction, choroid plexus bleed, ventricle filled with blood clots, periventricular hemorrhagic Infarction (PVHI), and hydrocephalus. There Is reduced myelination and axonal degeneration of the white matter. Macroscopically, blood clots can be visualized In the germinal matrix, ventricle, or brain parenchyma, depending on the severity of IVH. 10 Blood clots can often be seen In the cisterna magna and around the brain stem. Small clots confined to the germinal matrix are absorbed and are replaced with a small cyst. Resorption of blood clot takes several weeks and thus, It Is not seen In Infants who have lived for several months after the onset of IVH. Cavity(ies) has been demonstrated In the periventricular white matter of the Infants with moderate to large IVH, which Is secondary to PVHI. Dilated ventricle suggests post-hemorrhagic hydrocephalus (PHH). Microscopically, periventricular germinal zone and white matter shows areas of apoptosis, necrosis, cerebral edema as well as Infiltration of neutrophils, microglia and macrophages at an early stage. Often, a loss of ventricular and subventricular zone has been demonstrated more commonly on the lateral wall of the ventricle. Later, reactive astrocytes, hemosiderin-laden macrophages, and calcification of cells In white matter are noted. Immunostaining shows abundance of pre-myelinating oligodendrocyte progenitor cells (OPC), few mature oligodendrocytes, and reduced myelination of the white matter. Hemosiderin and Iron deposition can be demonstrated on Perls Prussian blue staining or immunolabeling.
PVHI has been reported In about 15% of Infant with IVH.11 It Is a venous Infarction of the periventricular white matter and Is associated with large IVH. PVHI are usually unilateral and asymmetrical Involving ventricular zone, subventricular zone, and adjacent white matter of the fronto-parietal lobe. These necrotic areas are subsequently converted Into porencephalic cyst(s). The pathogenesis of periventricular hemorrhagic Infarction (PVHI) is ascribed to the compression of terminal vein and its tributaries, which drains the cerebral white matter and germinal matrix. Periventricular leukomalacia (PVL) is frequently noted in infants with moderate to large IVH. In one series of cases of infants who died of IVH, PVL was reported in 75% of cases.10 PVL typically is an ischemic lesion, but is often seen in cases of IVH and could be hemorrhagic.
Mechanisms of disease
Extravasated blood components induces white matter injury and hydrocephalus in IVH.
IVH results in accumulation of blood into the cerebral ventricles and cisterna magna which leads to damage of the ventricular ependyma, white matter, and meninges. The initial injury is caused by plasma components of the blood, including thrombin, iron, immunoglobulin and complement. Later, the damage is aggravated by products of red blood cell (RBC) lysis, including hemoglobin and iron.12 There is also a mass effect by the blood clot compressing the wall of the ventricle. This elevates intracranial pressure, reduces the cerebral blood flow and cerebral oxygen metabolism, and disrupts the blood brain barrier permeability.
Among the blood components, thrombin, hemoglobin, and iron have been extensively studied as inducers of brain injury and hydrocephalus in animal models of IVH (Fig. 1).13, 14 Thrombin is a key player of blood coagulation cascade, but it also activates inflammation and stimulates protease activated receptors (PARs). PARs are expressed in OPCs; PAR1 inhibition reduces inflammation, promotes maturation of OPCs, and enhances myelination of the white matter in neonatal mouse models of brain injury.15, 16 Additionally, PAR-induced inflammation, gliosis, and extracellular matrix deposition contribute to the occurrence of hydrocephalus.17-19 Hemoglobin released by RBC lysis is scavenged by haptoglobin and endocytosed by macrophages; non-scavenged hemoglobin is metabolized to iron which initiates generation of free radicals, causing oxidative injury to neurons and glia.20, 21 Indeed, iron chelation therapy has reduced both white matter injury and hydrocephalus in neonatal and adult models of IVH.21
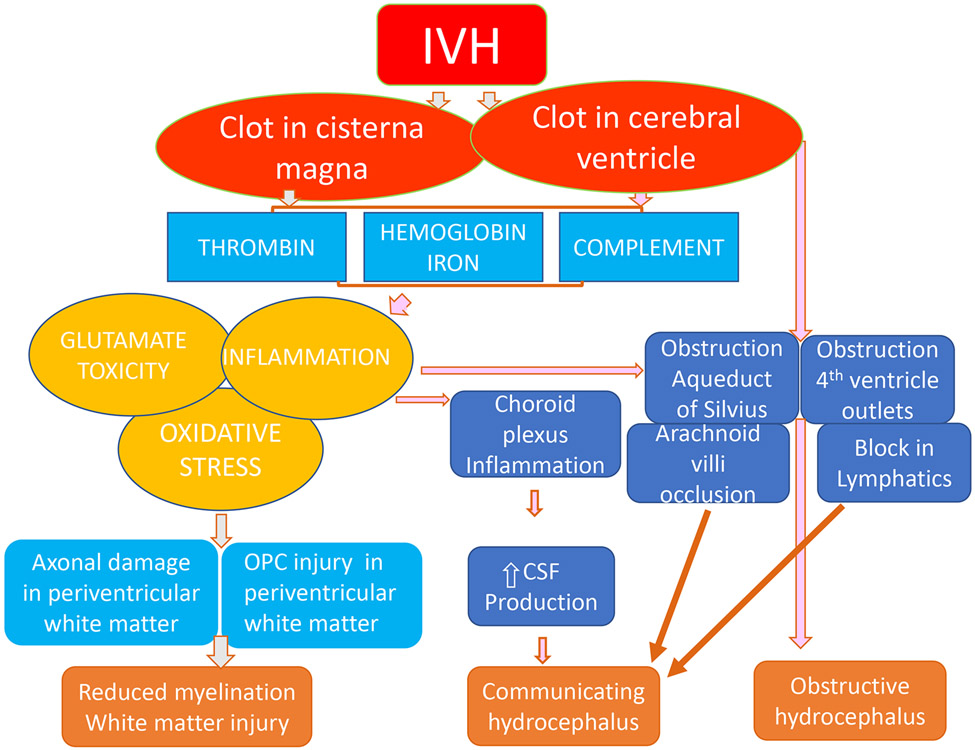
Figure 1: Mechanism of white matter injury and post-hemorrhagic hydrocephalus:
The schematic shows that IVH results in the accumulation of blood in the cerebral ventricle and cisterna magna. There is breakdown of the clot which results in release of hemoglobin, iron, thrombin, and complement. These blood components trigger oxidative stress, glutamate toxicity, and inflammation resulting in damage of the oligodendrocyte progenitors and hypomyelination. In addition, blood clots block the aqueduct of Sylvius, fourth ventricle outlets, lymphatics, and arachnoid villi hindering the flow of CSF and development of hydrocephalus. The inflammation of choroid plexus increases CSF production. CSF production. IVH: intra ventricular hemorrhage; CSF: cerebrospinal fluid; OPC: oligodendrocyte progenitor cells.
Pathogenesis of post-IVH hydrocephalus:
Post hemorrhagic hydrocephalus is either obstructive or communicating.22, 23 Obstructive hydrocephalus is acute and results from blood clots blocking the cerebral aqueduct of Sylvius or fourth ventricle outlets--foramen of Luschka or foramen of Magendie.22, 23 Communicating hydrocephalus, which is more common, results from increased cerebrospinal fluid (CSF) production, reduced CSF drainage, or both. The reduced CSF drainage is attributed to obliterative arachnoiditis occluding the arachnoid villi, lymphatic obstruction by the clots, inflammatory damage to the lymphatic network, and obliteration of CSF spaces around cranial nerves and blood vessels. However, lymphatics have been recently shown to be important portals of CSF drainage, and a network of lymphatics have been discovered in both dorsal and ventral dural meninges.24 Hence, lymphatics might be the key player in CSF drainage and their roles in the pathogenesis of post-hemorrhage hydrocephalus needs evaluation. CSF hypersecretion resulting from IVH-induced inflammation of the choroid plexus, as demonstrated in a rodent model, could be an important contributor of hydrocephalus in premature infants with IVH.25
To explain the pathogenesis of communicating hydrocephalus, bulk flow hypothesis and later hydrodynamic theory have been proposed.26 Bulk flow hypothesis states that CSF is produced in the choroid plexus and is transported by bulk flow to the arachnoid granulations at the venous sinuses for absorption. This theory has been criticized for a number of reasons: a) inability to explain CSF absorption in absence of arachnoid villi in preterm newborns, b) assuming that the choroid plexus in the sole site of CSF production, and c) CSF flow is linear, not pulsatile.26 Hydrodynamic theory postulates that dominant pulsatile CSF flow is responsible for the transport of CSF. These theories, although important for understanding the logistics of ventricular dilation as opposed to dilation of subarachnoid space in hydrocephalus, have not been thoroughly tested. Moreover, they do not directly provide a basis to develop mechanism-based strategies in the therapy of hydrocephalus.
The occurrence of IVH induces a robust inflammation and oxidative stress in the brain regions around the ventricle and in the meninges, which has been demonstrated in several animal models and autopsy material from premature infants. There is infiltration of neutrophils and microglia, production of pro-inflammatory cytokines, elevation in cyclo-oxygenase 2 (COX2) and prostaglandin E2 levels, apoptotic cell death, and astrogliosis. 27, 28, 29, 30 Glial cells produce cytokines, extracellular proteases, and reactive oxygen species. A number of signaling pathways are recruited, including Toll like receptors, nuclear factor kappa B (NF-kB), peroxisome proliferator-activated receptor gamma (PPARγ), signal transducer and activator of transcription (STAT), transforming growth factor-beta (TGFβ), mitogen-associated protein kinases (MAPK), and others. These signaling pathways contribute to inflammatory injury of the neural cells, astrogliosis, and remodeling of extracellular matrix in the periventricular brain region. Moreover, there is fibrosis and possibly damage of the meningeal capillaries, lymphatics, and intercellular stroma. The resultant pathological changes contribute to reduced CSF absorption and increased CSF production by the choroid plexus and cerebral capillaries leading to hydrocephalus.
Pathogenesis of white matter injury in infants with IVH:
The occurrence of IVH results in axonal injury and reduced myelination. Although there is evidence of axonal injury in IVH, the underlying molecular mechanisms leading to axonal damage have not been studied. Conversely, cellular and molecular mechanisms of myelination have been extensively studied in animal models of IVH. Oligodendrocytes are the neural cells that myelinate axons in the white matter. The OPCs are born and differentiate during 23-32 weeks of human gestation; infants born prematurely during this time window are at high risk of IVH.43, 44 Moreover, OPCs are born in the germinal matrix (called ganglionic eminence by neurobiologists), where IVH originates, making them vulnerable to blood-induced injury and death (Fig. 1). Studies on preterm rabbits have shown that the onset of IVH increases apoptosis, reduces proliferation, and inhibits maturation of OPCs.29, 31 Together, occurrence of IVH induces death and maturational failure of OPCs, causing hypomyelination of the white matter.
The development of IVH triggers inflammation, generation of reactive oxygen species, and glutamate excitotoxicity in the periventricular ganglionic eminence and adjacent white matter. With initiation of hemorrhage, the activated microglial cells and invading macrophages release pro-inflammatory cytokines, chemokines, and reactive oxygen and nitrogen species. In addition, glutamate in the extra-synaptic space accumulate owing to reduced capacity of the glial transporters to clear the glutamate, causing glutamate excitotoxicity and calcium-mediated injury of OPCs.32 Studies in animal models have shown that suppressing inflammation by COX2 or tumor necrosis factor-alpha (TNFα) inhibition restores myelination, suggesting that IVH-induced inflammation inhibits myelination.29 Likewise, reducing glutamate toxicity by inhibition of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors reduces OPC-injury and promotes myelination in animal models.33 Amongst the several potential sources of free radical generation, such as nicotinamide adenine dinucleotide phosphate, reduced (NADPH) oxidase, COX2, xanthine oxidase, nitric oxide synthase and mitochondria, NADPH oxidase has been demonstrated to be the main source of free-radical generation in a rabbit model of IVH.34 Accordingly, increased NADPH oxidase activity might contribute to OPC injury and myelination failure in premature infants with IVH.
A myriad of signaling pathways control production and maturation of oligodendrocytes. Of these, the key pathways are (bone morphogenetic protein (BMP), Notch, Wnt, and Sonic Hedge Hog.31, 35, 36 In addition, thyroid hormone signaling in the OPCs and hyaluronan levels in the extracellular matrix have important roles to play in OPC generation and maturation.30, 37 Studies in animal model of IVH have shown that BMP-2 and -4 are elevated in rabbit kits with IVH and they inhibit OPC maturation and myelination of the white matter. Lastly, thyroxine (T4) metabolism is dysregulated in the neural cells of infants with IVH. T4 is activated by deiodinase-2, which converts T4 into tri-iodothyronine (T3), while deiodinase 3 inactivates both T4 and T3. The cellular levels of thyroxine activating enzyme, deiodinase 2 is reduced, whereas thyroxine inactivating enzyme, deiodinase-3, is increased in brain samples of both humans and rabbits with IVH compared with controls without IVH.37 This leads to reduced thyroid hormone signaling in the neural cells of premature newborns with IVH, which has been rescued by thyroxine treatment in rabbit kits with IVH, resulting in improved myelination and neurologic recovery.
CLINCIAL COURSE AND OUTCOME
PHH in infants with IVH
About 20-25% of all infants with IVH develop PHH and 30-50% of infants with severe IVH develop post hemorrhagic ventricular dilatation (PVHD).38 A large multicenter study from NICHD participating NICUs has revealed that the incidence of shunt insertion in infants with IVH is 1, 1, 18 and 29% for grades I, II, III, and IV respectively.39 Increasing ventricular dilation adversely affects the neurodevelopmental outcome of infants with PHH. In a study of 139 infants with PHH, early intervention (ventricle size >97 percentile) by placement of ventricular reservoir or ventriculo-peritoneal (VP) shunt was compared with late surgical invention (ventricle size >97 percentile + 4 mm) and the results showed that motor, cognitive and language outcome was worse in late intervention group compared to early intervention.40 Multiple injury mechanisms, including stretch of axons, ischemia, hypoxia, inflammation, and demyelination, initiate the damage of the periventricular tissues with progressive enlargement of the lateral ventricle41, 42
The diagnosis of PHH is based on head ultrasound (Fig. 2). A number of diagnostic parameters are used by radiologists and neurosurgeons to diagnose and quantify the severity of PHH, which include Levene ventricular index (VI, the distance between the midline falx cerebri and the wall of the lateral ventricle at the level of foramen of Monro), anterior horn width (AHW, the distance between the medial wall and the floor of the lateral ventricle at the widest point), thalamo-occipital distance (TOD, the outermost point of the thalamus at its junction with the choroid plexus to the outermost part of the occipital horn) and frontal and occipital horn ratio ( FOHR, ratio of mean frontal and occipital horn dimension and biparietal dimension).
Figure 2: Grade III IVH resulting in post-hemorrhagic ventricular dilation:
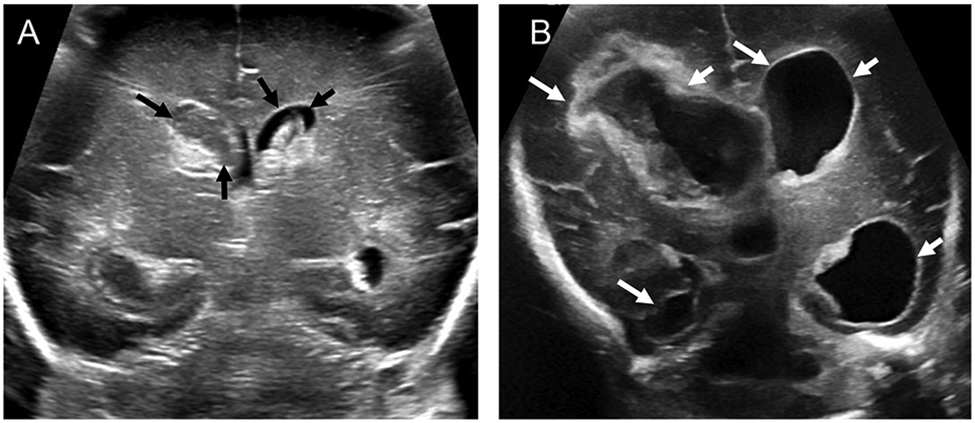
A) Head ultrasound (coronal view) shows bilateral grade III IVH (black arrows) in a preterm infant of 29 week gestational age at postnatal day 3. B) Ultrasound in coronal view of the same infants at 18 days of postnatal age shows dilatation of lateral ventricle (white arrows).
PHH progresses rapidly or slowly. In infants with slow progression of PHH, the ventricle size either regress or stabilize in about two-thirds of cases. Unfortunately, an increase in ventricle size is not proportionately reflected by elevation in the head circumference. This is related to high compliance and large extra-axial spaces of the brain of premature infants. It is, therefore, important to follow ventricular dimensions by neuroimaging in infants with IVH to assess PHVD.
Neurodevelopmental impairment in the preterm-born infants with IVH:
The severity of IVH and younger gestational age are independently associated with death and worse neurological outcomes. In a recent study on a large population (n=1472 survivors) of preterm infants (23-28 weeks), it has been reported that infants with grade III-IV IVH had higher rates of CP (30%) and developmental delay (17.5%) relative to infant with mild IVH or no IVH at 2 years of age.4 Additionally, infants with grade I-II IVH also had higher rates of CP (10.4% vs. 6.5%) and developmental delay (7.8 vs. 3.4%) relative to infants without IVH.4 Hence, neurodevelopmental impairments are higher even in infants with mild IVH compared to infants without IVH. These findings are in agreement with other major studies and a recent meta-analyses assessing the developmental outcome of infants with IVH.43 Studies evaluating long term developmental impact of IVH at 5-10 years of age have shown higher incidence of CP and cognitive impairment in both infants with mild and severe IVH compared to infants without IVH.44
The occurrence of IVH significantly increases the risk of neuro-psychiatric disorders. Accordingly, the incidence of attention deficit-hyperactivity, poor social skills, anxiety disorder, and depression, are higher in prematurely-born adolescents with IVH compared to prematurely-born adolescents who did not develop IVH.45 However, Isolated GMH-IVH does not increase the risk of autism spectrum disorder.46 The underlying mechanisms of psychiatric disorders in infants with IVH is not well understood; however, this might be related to both white matter and cortical injury in these infants.
Cranial ultrasound (CUS) is performed during the first week of life to detect IVH or white matter injury in premature infants of 30 weeks of gestational age or less. 47 In addition, head ultrasound or MRI brain scans at near-term age (35-42 weeks of postmenstrual age) is obtained to predict the risk of neurodevelopmental impairment.48, 49 The merits of CUS are that it is a bedside technique and allows serial imaging to assess the evolution of a lesion. The advantage of MRI scan includes detection of subtle white matter injury lesions—punctate lesions and diffuse extensive high signal intensity changes, which are missed in CUS. Moreover, several advanced MRI techniques, including diffusion tensor imaging (DTI), functional imaging (fMRI) and spectroscopy, and others, have been recently employed which offers more information about white matter development, connectivity, myelination, and metabolism in healthy and diseased brains. Together, IVH induces white matter injury in premature infants that can be detected with more precision using MRI compared to CUS.
Therapeutic Interventions for PHH
The treatment for PHH can be classified into medical treatment, repeated lumbar punctures, and neurosurgical management. Acetazolamide, a carbonic anhydrase inhibitor reduces CSF production by 30-60%. Loop diuretics, furosemide and bumetanide, in combination with acetazolamide or alone has reduced CSF production in canine and feline models.50 However, a randomized controlled trial on 177 infants with PHH showed that treatment using a combination of acetazolamide and furosemide resulted in no clinical benefit. Unexpectedly, the treatment led to higher rates of shunt surgery, death, and neurodevelopmental impairments in the treatment group of infants compared to controls51. Repeated lumbar puncture mitigates the increase in intracranial pressure, reduces ventriculomegaly and removes red blood cells and toxic blood products. However, four controlled randomized clinical trials showed that this approach does not reduce the risk for VP shunt surgery and developmental disabilities.52 In contradiction to these clinical trials, the early versus late ventricular intervention study (ELVIS) demonstrated that performing lumbar puncture during the correct window (VI >97percentile, but not at >97 percentile+ 4 mm) reduces a need for neurosurgical intervention in about 25% cases.53
Surgical management commonly performed in these infants includes placement of reservoirs (Ommaya or Rickham, Codman Shurtleff, MA, USA) subgaleal shunt, ventriculoperitoneal shunt, choroid plexus cauterization, and third ventriculostomy. Ventricular reservoirs are placed in smaller infants weighing usually less than 1.8 kg, which facilitates repeated tapping to prevent excessive head enlargement. Hence, this is a temporizing procedure because early insertion of VP shunt is associated with high rates of skin ulceration, infection, occlusion, and early revision owing to large amount of blood and protein in the CSF. A significant number of these infants do not need permanent shunt. VP shunts are performed in infants with reservoirs when there is progressive hydrocephalus with persistent need of tapping CSF in infants weighing 2 kg or more. Another temporary shunt procedure is subgaleal shunt, which uses a similar construct as ventricular reservoirs with 3-5 cm tubing attached to the reservoir outlet. The tubing is directed to a contralateral scalp pocket where CSF accumulates and gets reabsorbed. Choroid plexus cauterization and third ventriculostomy has been performed in a clinical trial in Uganda, in which a combination of endoscopy third ventriculostomy and choroid plexus cauterization yielded superior results compared to third ventriculostomy alone at one year of age.54
Since progression of PHH exacerbates white matter injury and reduces gray matter volume, it is important to make timely intervention. The ELVIS trial was conducted to compare the clinical outcomes of early (intervention at ventricular index >97 percentile) versus late intervention (intervention at ventricular index>97 percentile + 4 mm), in which interventions included CSF tapping by lumbar punctures followed by placement of reservoir.53 The results of that trial showed that there was no difference in the VP shunt placement or deaths between early (30%) and late (37%) intervention group.53 However, a VP shunt rate of 19% for grade III-IV IVH infants is the lowest rate reported so far. The follow up evaluation of these infants showed that a composite outcome of death and disability [CP, Bayley’s scales of infant development (BSID) cognitive, and/or motor score <70 BSID cognitive, and/or motor score<70] at 2 years of age was significantly better in the early intervention compared to the late intervention group, after adjusting for gestational age and severity of IVH.55 Moreover, the outcome was superior for those infants who did not develop PVHI and did not require CSF diversion surgery. Based on the experience with ELVIS trial, de Vries and other investigators have proposed a practical guideline to approach infants with PVHD.56 Briefly, infants with grade II-IV IVH should have biweekly CUS and head circumference measurements (Fig. 3). When VI exceeds 97th percentile in absence of a weekly increase in head circumference of more than 2 cm, lumbar puncture is recommended two-to-three times a week. The benefit of lumbar puncture is examined by CUS which is anticipated to stabilize ventricle dimensions. If there is no stabilization in the progression of ventriculomegaly, neurosurgical intervention is recommended. If VI is greater than 97th percentile plus 4 mm or weekly increase in head circumference is greater than 2 cm, the infant falls into high-risk category. The treatment for these infants is neurosurgical intervention—either temporizing measures (placement of reservoir or subgaleal shunt) or VP shunt. Infants in high-risk group might benefit from immediate lumbar puncture while the infant is awaiting surgery.
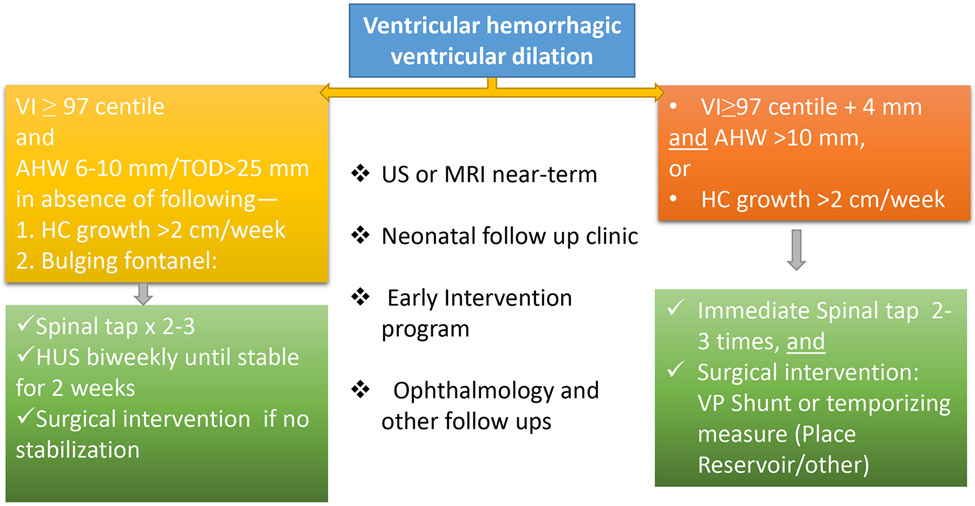
Figure 3: Suggested management of post hemorrhagic hydrocephalus.
The schematic shows an algorithm in management of post-IVH hydrocephalus, based on El-Dib et al 2020.56 VI: Levene ventricular index; AHW: anterior horn width; HC: head circumference; CUS: cranial ultrasound; VP: ventriculo-peritoneal.
Drainage, irrigation and fibrinolytic therapy (DRIFT) is based on the principle that elimination of products of blood clot lysis by using intraventricular (IV) fibrinolysis therapy and ventricle irrigation with artificial CSF would minimize the periventricular brain injury; thus it can reduce the incidence of PHH and neurodevelopment impairment in infants with IVH. In a randomized clinical trial of 77 infants across four centers, DRIFT did not significantly reduce the need for shunt surgery, however, severe cognitive disability (mental developmental index, MDI <55), measured by BSID evaluation at 2 years, was significantly reduced in treated infants compared to untreated controls.57 Unfortunately, DRIFT trial was stopped before the full planned recruitment. Together, refinement In the DRIFT, timely intervention of therapy in infants with PHH, and translating a number of preclinical studies into clinical trials might accelerate development of new therapies to improve the outcome of infants with PHH.
Therapeutic strategies to minimize white matter injury in infants with IVH.
Myelination is crucial for both efficient axonal conduction of the electrical impulses and preserving axonal integrity. The therapeutic strategies can be classified as
medical treatment
surgical drainage, irrigation and fibrinolytic therapy and
stem cell therapy
Medical treatment:
Alleviating iron and thrombin toxicity:
Iron chelation therapies have also conferred neuroprotection and reduced brain edema as well as hydrocephalus in a neonatal rat model of IVH.58 However, use of systemic or intracerebroventricular (ICV) deferoxamine may not be safe in premature infants.59 To alleviate thrombin-mediated toxicity on OPCs, dabigatran (thrombin inhibitor) and Vorapaxar (the PAR-1 receptor antagonist) have been evaluated in a neonatal rodent model of IVH.60 These treatments have reduced ventriculomegaly and have improved neurological outcome. PAR-1 inhibition reduces periventricular inflammation and enhances myelination in both neonatal and adult rodent model of ICH.15, 16, 17 Dabigatran is a FDA approved anticoagulant, however has a risk for exacerbating IVH. Vorapaxar has also been approved by the FDA in mid-2014 for the prevention of thrombotic complications in patients with myocardial infarction or peripheral artery disease. The TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome) clinical trial of vorapaxar in patients with acute coronary syndrome showed similar frequency of stroke for varapaxer versus placebo.61 Together, safety and efficacy of these treatments is a concern based on the available information in the preclinical models.
Inhibiting oxidative-inflammatory pathway:
Inflammation, oxidative stress, and glutamate excitotoxicity are important pathogenic events in IVH that are interlinked. Inhibiting these reactions are important strategies to reduce white matter injury in infant with IVH. Inhibition of inflammation diminishes oxidative stress and blocking glutamate receptor alleviates inflammation.29, 33, 34 Accordingly, COX2 inhibition by intramuscular (IM) celecoxib reduces pro-inflammatory cytokines, generation of reactive oxygen species, and microglia infiltration in a rabbit model of IVH.29 In addition, this treatment enhances myelination and neurologic recovery.41, 80 Celecoxib has adverse effects, including myocardial infarction, stroke, intestinal perforations, gastrointestinal bleeding. Concerns about myocardial infarction and stroke may not be pertinent in neonatal population. Celecoxib is one of the non-steroidal anti-inflammatory drugs (NSAID) that can be considered for a clinical trial because it has shown promise not only in animal models of IVH, but on other models of neonatal brain injury as well. Minocycline, a microglia inhibitor, has reduced ferritin level, brain edema, brain cell death, and hydrocephalus in rats pups with IVH relative to controls without IVH.58 Minocycline has undergone a clinical trial in adult patients with ischemic and hemorrhagic stroke and has shown efficacy and safety.62 However, these clinical trials were not adequately powered and thus, minocycline cannot be recommended for use in adult or neonatal patients with ICH. Toll-like receptor-4 (TLR4) is expressed in microglia and plays key roles in mediating inflammation. TLR4 inhibition reduces neuronal loss and edema formation and enhances neurological function.63 Moreover, in an adult rat model of IVH, both genetic and pharmacological inhibition of TLR4-NF-κB pathway reduces CSF production and ameliorates PHH.25
To reduce glutamate toxicity in infants with IVH, AMPA receptor inhibition has been used in a rabbit model of IVH.33 Both NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline) and perampanel treatment have enhanced myelination and neurological recovery in kits with IVH. In addition, AMPA receptor inhibition has offered neuroprotection in several models of brain injury, including hypoxia-ischemia, cerebral trauma, and multiple sclerosis. Major drawback of NBQX treatment is its renal toxicity. However, perampanel, a FDA-approved antiepileptic drug, has a low adverse-effect profile and an attractive pharmacokinetic profile, including a long half-life, allowing once daily dosing, and limited interaction to other antiepileptic drugs.64 Hence, perampanel is another neuroprotective agent which can possibly be evaluated in a clinical trial to improve outcome of infants with IVH.
Hormones and Growth factors:
Thyroxine treatment has improved myelination and neurological function in preterm rabbits with IVH.37 Moreover, thyroxine treatment promotes myelination in neonatal model of hypoxia-ischemia65 and a number of animal model of demyelination.66-68 Thyroxine treatment has been evaluated in premature infants in multiple clinical trials and found to be relatively safe.69, 70 Together, thyroxine treatment might enhance myelination and neurologic recovery in premature infants with IVH.
Epidermal growth factor (EGF) treatment has restored myelination and promoted clinical recovery in rabbit model of IVH and in models of demyelination as well as chronic hypoxia.71, 72 However, EGF might promote tumors and thus, investigators have raised doubts on its clinical use despite concrete demonstrations of efficacy in experimental conditions.73
Surgical treatment:
The DRIFT clinical trial showed an improvement in cognitive function in infants with IVH.57 However, there was an increase in the occurrence of secondary IVH and the clinical trial was prematurely stopped. It is possible that a refinement in the surgical procedure and timely performance of DRIFT might offer a better outcome to premature infants with IVH. In subsequent and recent studies, sophisticated neuro-endoscopic ventricular irrigation (NEL) was performed in infants with IVH, which led to significantly lower rates of permanent shunt placement and PHH.74, 75 However, these studies need to be conducted on an adequately powered sample size of infants to determine the efficacy and safety of this treatment in survivors with IVH.
Stem Cell treatment
An overwhelming number of studies have evaluated safety and efficacy of various types of stem cell therapy in animal models of brain injury.76 The beneficial effects of stem cell transplantation were attributed to engraftment of neural cells at the site of the lesion. However, recent studies showed that the transplanted cell die within a few weeks and neuroprotection is conferred by anti-inflammatory effects, secretion of tropic factors, stimulation of proliferation or maturation, transfer of healthy mitochondria to neural cells and others. Mesenchymal stem cells (MSCs) are most commonly used for clinical trials because of their safety profile, ease of isolation and propagation, and pleiotropic properties in alleviating organ damage. MSCs, thus, promote endogenous regeneration and recovery rather than substituting for dead neural cells.77
In a recent Korean study, injection of umbilical cord blood derived MSCs into the cerebral ventricles of rat model of IVH minimized apoptotic cell death, periventricular inflammation, and PHH as well as promoted myelination and neurologic recovery.78, 79 MSCs therapy has shown efficacy in neonatal animal models of hypoxia-ischemia.80 In a recent phase I clinical trial of intraventricular transplantation of MSCs in nine premature infants of ~26 weeks gestation with grade IV IVH, the treatment was well tolerated, and there were no serious adverse effects related to MSC transplantation.81 With the successful accomplishment of the phase I trial, a phase II trial has been started by the same group of investigators to determine the therapeutic efficacy of MSC treatment in survivors of IVH.81 The challenges in success of stem cell therapy are selection of the best type of stem cell, appropriate dose, correct route of administration, and right postnatal age of patient with moderate-to-severe IVH.
Together, stem cell therapy might show efficacy in clinical trials on preterm infants with moderate and severe IVH. However, the risk and benefits of stem cell therapy can only be assessed after the completion of the clinical trials.
Outlook.
The last two decades have witnessed a remarkable progress in the field of IVH and its complications, including white matter injury and PHH. This has come in part from the studies in animal models enhancing our understanding of the disease mechanism, advances in neuro-imaging, and clinical trials conducted on premature infants with IVH and PHH. All this information has positioned us to make sustained and accelerated progress in developing novel therapies for IVH-induced white matter injury and PHH. Therapeutic strategies focusing on elimination of blood products and reducing inflammation as well as oxidative stress, including DRIFT or endoscopic clot removal, iron chelation therapy, anti-thrombin treatment and/or stem cell transplantation, are likely to minimize both white matter injury and PHH. On the other hand, strategies to modulate oligodendrocyte maturation, for example thyroxine treatment, will promote myelination without significantly affecting PHH.
Infants with IVH exhibit a wide spectrum of severity and often manifest with a number of associated complications such as sepsis, necrotizing enterocolitis, patent ductus arteriosus, hypotension and others. Hence, an individualized approach has to be made in treatment selection. Grade I IVH infants have small risk of neurodevelopmental impairment and thus, these infants may not need any treatment in order to avoid adverse effects of a therapy. Select infants with grade II and those with grade III IVH might need a timely endoscopic removal of blood clot that may be followed with anti-inflammatory treatment. Isolated grade IV IVH cases with small amount of blood in the ventricle may not benefit from invasive surgery to remove the blood clot, but could show recovery with thyroxine, anti-inflammatory or anti-oxidative treatment. Despite a number of challenges inherent with these fragile infants, advances in understanding the mechanisms of disease and new clinical trials are likely to bring in novel therapies to minimize PHH and white matter injury in premature infants with IVH.
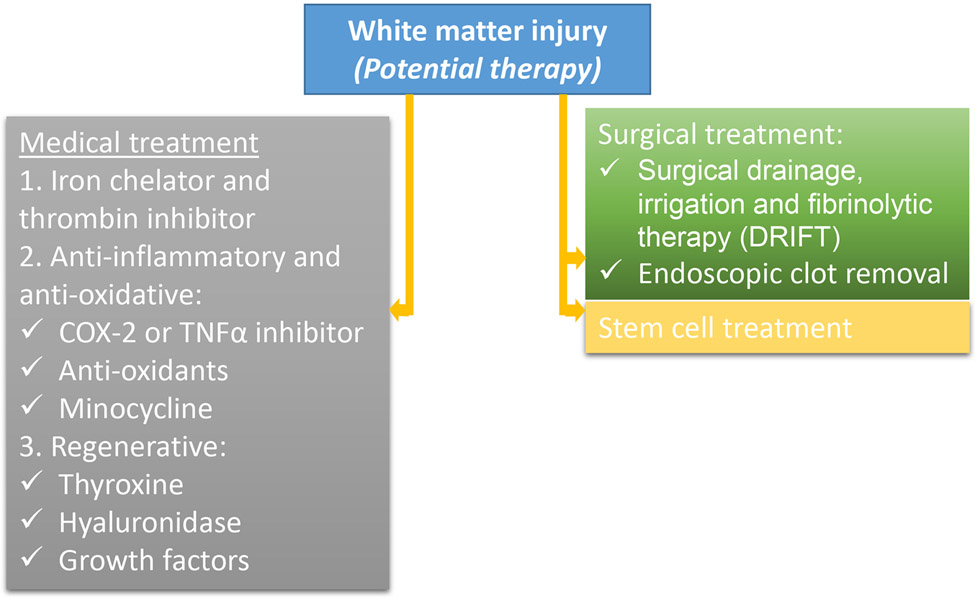
Figure 4. Potential therapeutic strategies for post-hemorrhagic white matter injury:
There is no proven treatment for post-hemorrhagic white matter injury and the schematic depicts therapies tested in preclinical studies and clinical trials. For additional details, please see text. COX: cyclo-oxygenase; TNFα: tumor necrosis factor alpha.
Acknowledgment:
I sincerely thank Dr. Jessica Kurian, MD from the Department of Radiology at Albert Einstein College of Medicine for providing the ultrasound images.
Footnotes
Conflict of interest: none
REFERENCES
- 1.Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002;110:143–151 [DOI] [PubMed] [Google Scholar]
- 2.McGowan EC, Vohr BR. Neurodevelopmental follow-up of preterm infants: What is new? Pediatric clinics of North America. 2019;66:509–523 [DOI] [PubMed] [Google Scholar]
- 3.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. The Journal of pediatrics. 1978;92:529–534 [DOI] [PubMed] [Google Scholar]
- 4.Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133:55–62 [DOI] [PubMed] [Google Scholar]
- 5.Davis AS, Hintz SR, Goldstein RF, Ambalavanan N, Bann CM, Stoll BJ, et al. Outcomes of extremely preterm infants following severe intracranial hemorrhage. J Perinatol. 2014;34:203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne AH, Hintz SR, Hibbs AM, Walsh MC, Vohr BR, Bann CM, et al. Neurodevelopmental outcomes of extremely low-gestational-age neonates with low-grade periventricular-intraventricular hemorrhage. JAMA pediatrics. 2013;167:451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340–346 [DOI] [PubMed] [Google Scholar]
- 8.Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, Murray RM. Impaired executive functioning in young adults born very preterm. Journal of the International Neuropsychological Society : JINS. 2007;13:571–581 [DOI] [PubMed] [Google Scholar]
- 9.Indredavik MS, Skranes jS, Vik T, Heyerdahl S, Romundstad P, Myhr GE, et al. Low-birth-weight adolescents: Psychiatric symptoms and cerebral mri abnormalities. Pediatr Neurol. 2005;33:259–266 [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DL, Sauls CD, Goddard-Finegold J. Neuropathologic findings in short-term survivors of intraventricular hemorrhage. Am J Dis Child. 1987;141:617–621 [DOI] [PubMed] [Google Scholar]
- 11.Volpe JJ. Edward b. Neuhauser lecture. Current concepts of brain injury in the premature infant. AJR Am J Roentgenol. 1989;153:243–251 [DOI] [PubMed] [Google Scholar]
- 12.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke. 2011;42:1781–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63 [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264 [DOI] [PubMed] [Google Scholar]
- 15.Yoon H, Radulovic M, Drucker KL, Wu J, Scarisbrick IA. The thrombin receptor is a critical extracellular switch controlling myelination. Glia. 2015;63:846–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, et al. Blood-brain barrier breakdown and repair by src after thrombin-induced injury. Annals of neurology. 2010;67:526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos-Mandujano G, Vazquez-Juarez E, Hernandez-Benitez R, Pasantes-Morales H. Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia. 2007;55:917–925 [DOI] [PubMed] [Google Scholar]
- 18.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48:1592–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu DZ, Sharp FR. The dual role of src kinases in intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:77–81 [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1243–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda T, Hida H, Kanda Y, Aihara N, Ohta K, Yamada K, et al. Oral administration of metal chelator ameliorates motor dysfunction after a small hemorrhage near the internal capsule in rat. J Neurosci Res. 2007;85:213–222 [DOI] [PubMed] [Google Scholar]
- 22.Hill A, Shackelford GD, Volpe JJ. A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Pediatrics. 1984;73:19–21 [PubMed] [Google Scholar]
- 23.Larroche JC. Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biol Neonate. 1972;20:287–299 [DOI] [PubMed] [Google Scholar]
- 24.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23:997–1003 [DOI] [PubMed] [Google Scholar]
- 26.Greitz D. The hydrodynamic hypothesis versus the bulk flow hypothesis. Neurosurg Rev. 2004;27:299–300 [DOI] [PubMed] [Google Scholar]
- 27.Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, et al. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39:3378–3388 [DOI] [PubMed] [Google Scholar]
- 28.Xue M, Balasubramaniam J, Buist RJ, Peeling J, Del Bigio MR. Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J Neuropathol Exp Neurol. 2003;62:1154–1165 [DOI] [PubMed] [Google Scholar]
- 29.Vinukonda G, Csiszar A, Hu F, Dummula K, Pandey NK, Zia MT, et al. Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-alpha inhibition. Brain : a journal of neurology. 2010;133:2264–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinukonda G, Dohare P, Arshad A, Zia MT, Panda S, Korumilli R, et al. Hyaluronidase and hyaluronan oligosaccharides promote neurological recovery after intraventricular hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:872–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dummula K, Vinukonda G, Chu P, Xing Y, Hu F, Mailk S, et al. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:12068–12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070 [DOI] [PubMed] [Google Scholar]
- 33.Dohare P, Zia MT, Ahmed E, Ahmed A, Yadala V, Schober AL, et al. Ampa-kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:3363–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zia MT, Csiszar A, Labinskyy N, Hu F, Vinukonda G, Lagamma EF, et al. Oxidative-nitrosative stress in a rabbit pup model of germinal matrix hemorrhage: Role of nad(p)h oxidase. . Stroke 2009. 40 2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dohare P, Cheng B, Ahmed E, Yadala V, Singla P, Thomas S, et al. Glycogen synthase kinase-3beta inhibition enhances myelination in preterm newborns with intraventricular hemorrhage, but not recombinant wnt3a. Neurobiology of disease. 2018;118:22–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergles DE, Richardson WD. Oligodendrocyte development and plasticity. Cold Spring Harb Perspect Biol. 2015;8:a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vose LR, Vinukonda G, Jo S, Miry O, Diamond D, Korumilli R, et al. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17232–17246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellenbogen JR, Waqar M, Pettorini B. Management of post-haemorrhagic hydrocephalus in premature infants. J Clin Neurosci. 2016;31:30–34 [DOI] [PubMed] [Google Scholar]
- 39.Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R, Network NR. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 2008;121:e1167–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasakumar P, Limbrick D, Munro R, Mercer D, Rao R, Inder T, et al. Posthemorrhagic ventricular dilatation-impact on early neurodevelopmental outcome. Am J Perinatol. 2013;30:207–214 [DOI] [PubMed] [Google Scholar]
- 41.Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain pathology. 2004;14:317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAllister JP 2nd. Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med. 2012;17:285–294 [DOI] [PubMed] [Google Scholar]
- 43.Mukerji A, Shah V, Shah PS. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: A meta-analysis. Pediatrics. 2015;136:1132–1143 [DOI] [PubMed] [Google Scholar]
- 44.Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Pehboeck-Walser N, Fussenegger B. Early risk predictors for impaired numerical skills in 5-year-old children born before 32 weeks of gestation. Acta Paediatr. 2013;102:66–71 [DOI] [PubMed] [Google Scholar]
- 45.Whitaker AH, Feldman JF, Lorenz JM, McNicholas F, Fisher PW, Shen S, et al. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Arch Gen Psychiatry. 2011;68:742–752 [DOI] [PubMed] [Google Scholar]
- 46.Movsas TZ, Pinto-Martin JA, Whitaker AH, Feldman JF, Lorenz JM, Korzeniewski SJ, et al. Autism spectrum disorder is associated with ventricular enlargement in a low birth weight population. The Journal of pediatrics. 2013;163:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: Neuroimaging of the neonate: Report of the quality standards subcommittee of the american academy of neurology and the practice committee of the child neurology society. Neurology. 2002;58:1726–1738 [DOI] [PubMed] [Google Scholar]
- 48.Spittle AJ, Cheong J, Doyle LW, Roberts G, Lee KJ, Lim J, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev Med Child Neurol. 2011;53:1000–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal mri to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694 [DOI] [PubMed] [Google Scholar]
- 50.Javaheri S, Wagner KR. Bumetanide decreases canine cerebrospinal fluid production. In vivo evidence for nacl cotransport in the central nervous system. J Clin Invest. 1993;92:2257–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy CR, Ayers S, Campbell MJ, Elbourne D, Hope P, Johnson A. Randomized, controlled trial of acetazolamide and furosemide in posthemorrhagic ventricular dilation in infancy: Follow-up at 1 year. Pediatrics. 2001;108:597–607 [DOI] [PubMed] [Google Scholar]
- 52.Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. The Cochrane database of systematic reviews. 2001:CD000216. [DOI] [PubMed] [Google Scholar]
- 53.de Vries LS, Groenendaal F, Liem KD, Heep A, Brouwer AJ, van 't Verlaat E, et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: A randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019;104:F70–F75 [DOI] [PubMed] [Google Scholar]
- 54.Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: A prospective study in 550 african children. Journal of neurosurgery. 2005;103:475–481 [DOI] [PubMed] [Google Scholar]
- 55.Cizmeci MN, Groenendaal F, Liem KD, van Haastert IC, Benavente-Fernandez I, van Straaten HLM, et al. Randomized controlled early versus late ventricular intervention study (elvis) in posthemorrhagic ventricular dilatation: Outcome at 2 years. The Journal of pediatrics. 2020 [DOI] [PubMed] [Google Scholar]
- 56.El-Dib M, Limbrick DD Jr., Inder T, Whitelaw A, Kulkarni AV, Warf B, et al. Management of post-hemorrhagic ventricular dilatation in the infant born preterm. The Journal of pediatrics. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: Developmental outcome at 2 years. Pediatrics. 2010;125:e852–858 [DOI] [PubMed] [Google Scholar]
- 58.Guo J, Chen Q, Tang J, Zhang J, Tao Y, Li L, et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain Res. 2015;1594:115–124 [DOI] [PubMed] [Google Scholar]
- 59.deLemos RA, Roberts RJ, Coalson JJ, deLemos JA, Null DM Jr., Gerstmann DR. Toxic effects associated with the administration of deferoxamine in the premature baboon with hyaline membrane disease. Am J Dis Child. 1990;144:915–919 [DOI] [PubMed] [Google Scholar]
- 60.Klebe D, Flores JJ, McBride DW, Krafft PR, Rolland WB, Lekic T, et al. Dabigatran ameliorates post-haemorrhagic hydrocephalus development after germinal matrix haemorrhage in neonatal rat pups. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37:3135–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ungar L, Clare RM, Rodriguez F, Kolls BJ, Armstrong PW, Aylward P, et al. Stroke outcomes with vorapaxar versus placebo in patients with acute coronary syndromes: Insights from the tracer trial. Journal of the American Heart Association. 2018;7:e009609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malhotra K, Chang JJ, Khunger A, Blacker D, Switzer JA, Goyal N, et al. Minocycline for acute stroke treatment: A systematic review and meta-analysis of randomized clinical trials. J Neurol. 2018;265:1871–1879 [DOI] [PubMed] [Google Scholar]
- 63.Lin S, Fan LW, Rhodes PG, Cai Z. Intranasal administration of igf-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Experimental neurology. 2009;217:361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogawski MA. Revisiting ampa receptors as an antiepileptic drug target. Epilepsy Curr. 2011;11:56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schang AL, Gressens P, Fleiss B. Revisiting thyroid hormone treatment to prevent brain damage of prematurity. J Neurosci Res. 2014;92:1609–1610 [DOI] [PubMed] [Google Scholar]
- 66.Calza L, Fernandez M, Giuliani A, Aloe L, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and ngf content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 2002;99:3258–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franco PG, Silvestroff L, Soto EF, Pasquini JM. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Experimental neurology. 2008;212:458–467 [DOI] [PubMed] [Google Scholar]
- 68.Harsan LA, Steibel J, Zaremba A, Agin A, Sapin R, Poulet P, et al. Recovery from chronic demyelination by thyroid hormone therapy: Myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:14189–14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.La Gamma EF, van Wassenaer AG, Ares S, Golombek SG, Kok JH, Quero J, et al. Phase 1 trial of 4 thyroid hormone regimens for transient hypothyroxinemia in neonates of <28 weeks' gestation. Pediatrics. 2009;124:e258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Wassenaer AG, Kok JH, de Vijlder JJ, Briet JM, Smit BJ, Tamminga P, et al. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks’ gestation. N Engl J Med. 1997;336:21–26 [DOI] [PubMed] [Google Scholar]
- 71.Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11092–11106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scafidi J, Hammond TR, Scafidi S, Ritter J, Jablonska B, Roncal M, et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506:230–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berlanga-Acosta J, Gavilondo-Cowley J, Lopez-Saura P, Gonzalez-Lopez T, Castro-Santana MD, Lopez-Mola E, et al. Epidermal growth factor in clinical practice - a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6:331–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etus V, Kahilogullari G, Karabagli H, Unlu A. Early endoscopic ventricular irrigation for the treatment of neonatal posthemorrhagic hydrocephalus: A feasible treatment option or not? A multicenter study. Turk Neurosurg. 2018;28:137–141 [DOI] [PubMed] [Google Scholar]
- 75.Schulz M, Buhrer C, Pohl-Schickinger A, Haberl H, Thomale UW. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. Journal of neurosurgery. Pediatrics 2014;13:626–635 [DOI] [PubMed] [Google Scholar]
- 76.Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, Heijnen C, et al. Stem cell therapy for neonatal brain injury: Perspectives and challenges. Annals of neurology. 2011;70:698–712 [DOI] [PubMed] [Google Scholar]
- 77.Chang YS, Ahn SY, Sung S, Park WS. Stem cell therapy for neonatal disorders: Prospects and challenges. Yonsei Med J. 2017;58:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahn SY, Chang YS, Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J Pediatr. 2014;57:251–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahn SY, Chang YS, Sung DK, Sung SI, Yoo HS, Lee JH, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44:497–504 [DOI] [PubMed] [Google Scholar]
- 80.Kim ES, Ahn SY, Im GH, Sung DK, Park YR, Choi SH, et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res. 2012;72:277–284 [DOI] [PubMed] [Google Scholar]
- 81.Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: Phase i dose-escalation clinical trial. Stem Cells Transl Med. 2018;7:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]