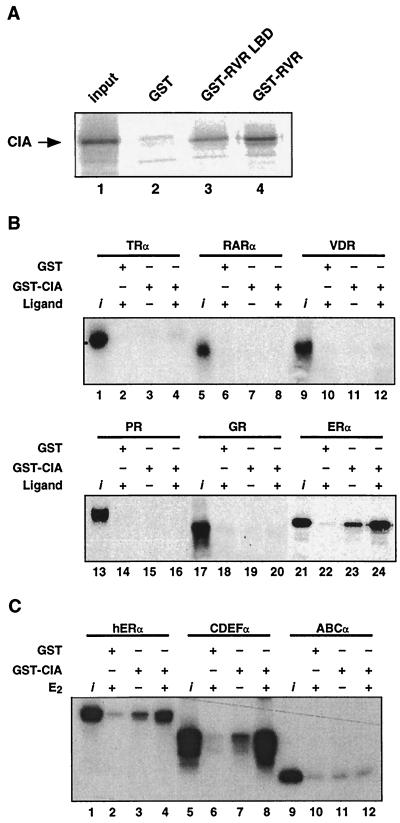
FIG. 3.
CIA in vitro interaction shows specificity for RVR and ERα. (A) [35S]methionine-labeled CIA interacts in vitro with the GST-RVR LBD (lane 3) and full-length GST-RVR (lane 4) but not with the GST control protein (lane 2). The input represents 10% of the labeled CIA used in the assay (lane 1). (B) [35S]methionine-labeled ERα specifically interacts in both ligand-independent and -dependent fashions with GST-CIA. The assay was performed in the presence of either 10−7 M E2 (lanes 22 and 24) or the vehicle (ethanol, lane 23). None of the other nuclear receptors tested, including TRα, RARα, VD3R, PR, and GR, demonstrated any interaction with GST-CIA (lanes 1 to 20) either in the absence or in the presence of the appropriate ligands (T3Rα, 3-iodothyronine at 10−6 M; RARα, all-trans retinoic acid at 10−6 M; VD3R, 1,25-dihydroxyvitamin D3 at 10−7 M; PR, progesterone at 10−7 M; GR, dexametasone at 10−7 M). (C) Interaction between CIA and ERα requires the C-terminal portion of ERα. Full-length [35S]methionine-labeled ERα proteins and the same protein with the amino-terminal domain truncated (CDEFα) or the carboxy-terminal domain truncated (ABCα) were incubated with GST-CIA. Pull-down assays were performed as described in Materials and Methods.