Abstract
Mesenchymal stem cells (MSCs) have long been regarded as critical components of regenerative medicine strategies, given their multipotency and persistence in a variety of tissues. Recently, the specific role of MSCs in mediating regenerative outcomes has been attributed (in part) to secreted factors from transplanted cells, namely extracellular vesicles. This viewpoint manuscript highlights the promise of cell-derived extracellular vesicles as agents of regeneration, enhanced by synergy with appropriate biomaterials platforms. Extracellular vesicles are a potentially interesting regenerative tool to enhance the synergy between MSCs and biomaterials. As a result, we believe these technologies will improve patient outcomes through efficient therapeutic strategies resulting in predictable patient outcomes.
Keywords: Biomaterials, Extracellular vesicles, Mesenchymal stem cells, Regenerative medicine, Tissue engineering
Introduction
Originally conceived to solve the shortage of organs for transplantation, the field of tissue engineering has evolved to encompass a broad clinical scope including regeneration of simple and complex tissues in a variety of clinical settings (Langer and Vacanti, 1993). At their core, tissue engineering strategies rely on three tenants: isolated cells, inductive substances, and matrices to facilitate organization, largely biomaterials (Khademhosseini and Langer, 2016; Langer and Vacanti, 2016). Despite significant academic advances, clinical translation remains slow due to challenges concerning cell sourcing, manufacturing scale, standardization, and regulation (Hoffman et al., 2019).
Mesenchymal stromal cells (MSCs) have attracted significant attention as an ideal multipotent stem cell source since their discovery as fibroblast-colony forming cells (Friedenstein et al., 1970). MSCs are extracted from a variety of tissue sources and are capable of multilineage differentiation (Yingst and Hoffman, 1984). Over 800 clinical trials have been conducted to determine their therapeutic efficacy (Kabat et al., 2019; Squillaro et al., 2016). However, no MSC therapies have been formally approved for use in the United States Food and Drug Administration. Significant concerns around the large-scale preparation of MSCs remains challenging (Jayaraman et al., 2021; Phinney and Galipeau, 2019; Sensebé et al., 2013).
Concurrent with advances in tissue engineering, advances in molecular and developmental biology have significantly informed innovative tissue engineering strategies (Lenas, 2018). In this viewpoint we highlight recent advances in investigational therapeutics which pose significant translational advantages using extracellular vesicles as agents of regeneration, in novel combination with biomaterial platforms, illustrated in Fig. 1. We hypothesize that thoughtfully designed biomaterials paired with cell-instructive signals may induce predictable regeneration by endogenous cell sources, posing significant translational advantages as next generation tissue engineering therapeutics.
FIGURE 1.
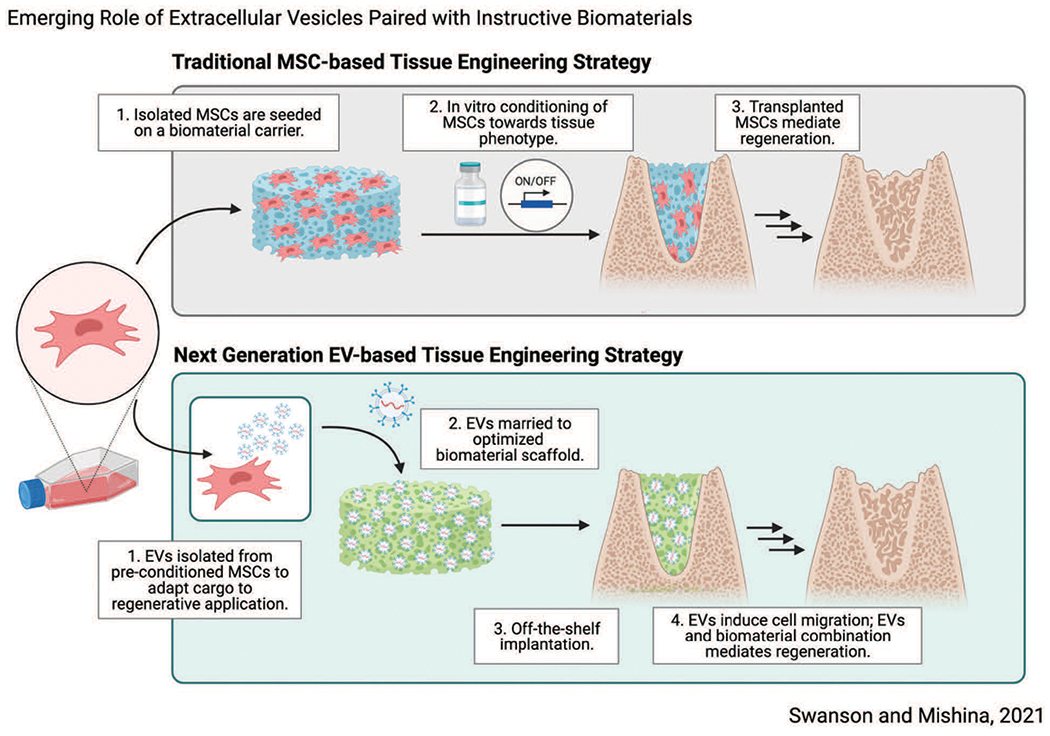
Schematic overview demonstrating next-generation tissue engineering therapeutic strategy which relies on synergy between biomaterial scaffolds and sustained release of EVs to induce tissue regeneration. Made with Biorender.
Biomaterials Modulate Cell and Tissue Fate
MSCs respond to physical, chemical, and mechanical environment, providing a role for biomaterials-instructed regeneration (Jang and Kim, 2010; Leach and Whitehead, 2018). In addition to providing tissue organization in three dimensions, biomaterial features play a role in determining tissue fate through porosity (Loh and Choong, 2013), stiffness (Breuls et al., 2008), texture (Smith et al., 2009; Zhang and Ma, 2000), pore size (Gupte et al., 2018; Swanson et al., 2021), and chemical functionality (Zou et al., 2018), with the goal of replicating the niche or microenvironment of target cells and tissues to increase regenerative success (Williams, 2019). Biomaterials may be impregnated with growth factors or controlled release moieties to display inductive signals to cells, mimicking in vitro administration and secretion in vivo, which increases efficiency and minimizes off-target effects (Swanson et al., 2020b; Swanson et al., 2020c). Decellularized biomaterial matrices, containing residual proteins, are approved by the FDA in various forms and provide inspiration for a combination of inductive cue display within a biomaterial (Schmidt, 2012). Synthetic materials offer a greater degree of design tunability and manufacturing advantages (Agmon and Christman, 2016; Swanson and Ma, 2020); their fabrication methods are highly scalable, representing a clear path to clinical scale which is more cost-effective than cell-based therapies (Greenberg-Worisek et al., 2018; Sanz-Nogués and O’Brien, 2021; ten Ham et al., 2020).
Secreted Factors Enhance Biomaterials-Based Regeneration
Kitami et al. (2016) demonstrate that prolonged survival of transplanted cells does not directly accelerate osseous wound healing, despite accelerated healing in defects treated with cells (Kitami et al., 2016). These results suggest that transplanted cells alone are not responsible for regenerative outcomes directly, yet they provide important instructive signals. Similar findings in transplanted adipose-derived MSCs have been reported (Muhammad et al., 2017). The secretome, the composite milieu of cells’ secreted factors which includes: proteins, growth factors, and extracellular vesicles (EVs), has recently been identified as a critical driver of cell fate (Pinho et al., 2020). Saha et al. (2019) demonstrated similar results in the functional recovery of ischemic myocardium after cardiac progenitor cell (CPC) transplantation, and specifically identified EVs produced by CPCs, one component of cellsecreted factors that is readily isolated, contained microRNAs associated with myocardial recovery. These findings suggest that transplanted cells may act as an in-situ drug factory, synthesizing inductive cues which catalyze regeneration, rather than directly participate (Moghadasi et al., 2021). In the context of tissue engineering, it is plausible to replace transplanted MSCs with secreted factors, such as EVs, in a way which mimics their natural secretion (Fig. 1).
Growing Role for Extracellular Vesicles in Catalyzing Regeneration
EVs are lipid-bound vesicles with diameters in the range of 50–150 nm (Swanson et al., 2020a; Thery et al., 2018; Witwer et al., 2019). Originally thought to be a waste shedding mechanism by cells, recent evidence suggests that EVs are nature’s endogenous nanoparticle delivery system and a form of cell-cell communication, containing microRNAs and proteins (van Niel et al., 2018). Like stem cells, EVs have shown important therapeutic potentials in a variety of disease states and target tissues, outlined in Table 1.
TABLE 1.
Diverse demonstrations of various EV-based therapeutic applications selected from the literature
| Regenerative target | Donor cell | Reference |
|---|---|---|
| Bone Mineralization | Bone Marrow MSCs | (Narayanan et al., 2016) |
| Mineralizing Osteoblasts | (Cui et al., 2016) | |
| Osteoclasts | (Huynh et al., 2016) | |
| Adipose Derived MSCs | (An et al., 2019) | |
| Bone Angiogenesis | Umbilical-cord Derived MSCs | (Zhang et al., 2012) |
| Induced Pluripotent SC-derived MSCs | (Hu et al., 2015) | |
| Intervertebral Disk Degeneration | Bone Marrow MSC, Nucleus Pulposus Cells, Adipose Stem Cells | (DiStefano et al., 2021) |
| Cardiac Ventricular Remodeling | C2C12 Myoblasts | (Yamaguchi et al., 2015) |
| Cardiosphere-derived Cells | (Ibrahim et al., 2014) | |
| Lung | Bone Marrow MSCs | (Lee et al., 2012) |
| Kidney | Bone Marrow MSCs | (Zhou et al., 2013) |
| Brain | Dendritic Cells | (Alvarez-Erviti et al., 2011) |
| Brain (Alzheimer’s Dz) | Murine Neuroblastoma Neuro2a Cells | (Yuyama et al., 2014) |
| Peripheral Nerve Repair | Adipose Derived MSCs | (Ching and Kingham, 2015) |
| Schwann Cells | (Ching and Kingham, 2015) | |
| Cutaneous Wound Healing | Epidermal SC | (Duan et al., 2020) |
| Bone Marrow MSCs | (Ha et al., 2020) | |
| Cartilage | Bone Marrow MSC | (Chen et al., 2018; Tan et al., 2021) |
| Gingival Mucosa | Gingival MSC | (Shi et al., 2017) |
Note this is not an exhaustive list, many of these examples demonstrate in vitro or preliminary in vivo utility and serve as a basis for future investigation in the context of tissue engineering. Note this is not an exhaustive list, but aims to demonstrate breadth.
EV-based therapeutics are promising regarding their translational and therapeutic potential. Ibrahim et al. (2014) isolated cardiosphere-derived cell EVs and profiled their molecular cargo to determine enriched miRNAs after demonstrating EV injection recapitulates the regenerative effects of transplanted cells. Inhibition of EV biosynthesis in vivo blocked these same effects. Interestingly, administration of the upregulated miR-146a reproduced only some, but not all, effects of EV administration. The authors propose EVs as a method of tying together regenerative paracrine and autocrine effects of cardiac progenitors without manually postulating their complex mixtures of signaling molecules.
The molecular cargo of EVs is reflective of its donor cell identity, and culture environment (Dai et al., 2019; Fevrier and Raposo, 2004; Quesenberry and Aliotta, 2010). This affords significant, large-scale cell culture manipulations to take place in vitro which tailor EV cargo towards specific regenerative applications, for example, by small molecule or growth factor treatment. It is also reasonable to consider biomaterial culture platforms as a method of large-scale EV manufacturing, given our understanding of biomaterial influences on cell phenotype. 3D cultures are also shown to increase EV yield in response to tissue-like organization (Lee et al., 2021; Rocha et al., 2019). Additionally, EVs isolated from highly controlled culture systems may be optimally tuned to educate naïve recipient cells (endogenous or transplanted) in recipient tissue defects, minimizing the requirement of preconditioned cells for transplantation.
Compared to MSCs, EVs exhibit “immune privilege” and demonstrate a better safety profile in terms of tumorigenicity and immunogenicity (Rani et al., 2015; Zhang et al., 2018b). EVs are shown to be well-tolerated without adverse immune responses or need for immunosuppressive agents (Mendt et al., 2018). EVs from immortalized cell lines represent an opportunity to standardize their biosynthesis and cargo (Deb et al., 2019; Kim et al., 2021; Swanson et al., 2020b) given that immortalized cells are less susceptible to change over time. Recombinant DNA technology may allow for further manipulation of the EV membrane or cargo, recently described as “designer exosomes” (Jafari et al., 2020). Recent literature suggests cross-species efficacy of EVs (Swanson et al., 2020b; Swanson et al., 2020c; Zhu et al., 2017); plant-derived EVs are also under investigation for various therapeutic uses (Akuma et al., 2019; Garaeva et al., 2021). As a result of recent interest in EV-based therapeutics, good manufacturing practices (GMP) have been developed for their commercial manufacturing (Bahr et al., 2020; Colao et al., 2018; Harn et al., 2020; Mendt et al., 2018).
The ideal regenerative therapeutic would allow for off-the-shelf clinical use and require minimal preparation, particularly for routine applications such as in clinical dentistry and dermatology. Researchers must consider that most healthcare settings do not have advanced tissue culture capability to handle or culture MSCs for use in tissue engineering applications, when required. Compared to MSCs, EVs are easily lyophilized and stored for future use (El Baradie et al., 2020; Swanson et al., 2020b). Charoenviriyakul et al. (2018) demonstrated that lyophilized EVs retained their activity for approximately 4 weeks even when stored at 25°C (room temperature), which poses significant clinical and commercial distribution advantages.
Vision for Next-Generation Regenerative Technology
Despite numerous human clinical trials underway with EV-based therapeutics for a variety of clinical applications, most are limited to intravenous infusion or direct injection. EVs circulate the body rapidly, thereby requiring a high dose to reach therapeutic efficacy and pose risk for off-target effects. In the context of tissue engineering, the therapeutic effect is needed and desired locally. Our group and others have reported early developments in the delivery and sustained release of EVs by clinically and biologically relevant means. An important feature of these biomaterials platforms is that they are highly versatile. EV cargo may be changed (see Table 1 for examples) based on the clinical indication and desired outcomes, however the design of the platform technology remains otherwise unchanged. This allows for versatile and widespread use of these biomaterials technologies as platform technologies.
Hydrogels encapsulating EVs function to maintain EVs at the site of implantation, increasing their half-life in vivo (Zhang et al., 2018a). Historically hydrogels have had mixed success with the long-term encapsulation of cells due to mass transfer limitations. Because EVs are non-living, many fewer parameters must be considered. Gingival MSC in chitosan/silk hydrogel sponge accelerates wound healing on skin defects in diabetic mice by inducing neoepithelialization and angiogenesis to a greater degree than the hydrogel alone (Shi et al., 2017). Other examples of hydrogel-based EV delivery are discussed by Riau et al. (2019).
Synthetic biodegradable materials which encapsulate EVs in controlled amounts allow for their controlled dosing and long-term sustained release. We demonstrated the first report of an EV-containing poly(lactic acid-co-glycolic acid) (PLGA) microsphere. Over time, the PLGA polymer is degraded to allow EV release to local cells. We demonstrated that this delivery system was sufficient to induce odontogenesis (mineralized dentin formation) as a novel pulp-capping strategy to protect vital tooth tissue, where EV or cell administration would be otherwise limited. In this way, EVs are locally released from a depot for up to 12 weeks (Swanson et al., 2020b). As a further development of this technology, we developed a microsphere delivery platform which can be embedded into a tissue engineering scaffold. This approach combines the advantageous properties of EVs and their sustained release with a biomaterial scaffold optimized for bone regeneration (Swanson et al., 2020c). We demonstrated that this approach was sufficient to catalyze osseous wound healing of a calvarial defect without the transplantation of exogenous MSCs. Instead, we relied on released EVs to guide the fate of endogenous cells. We anticipate that these technologies are key to clinical translation of regenerative EV therapeutics. Other motifs of EV tethering, including ECM-inspired immobilization, covalent conjugation, and electrostatic interaction are described by Man et al. (2020).
Comparisons of MSC-based and EV-based regenerative technology consider that MSC sources are well-characterized and readily accessible (Moghadasi et al., 2021). While cell populations involved in tissue formation and repair are characterized for many tissues, ideal progenitor populations remain elusive for others or may not be suitable to autologous expansion and re-implantation. In these cases, EVs may be advantageous in that they can be produced at a larger scale than the cell source itself, and EVs from cell sources other than the target source may be able to catalyze regenerative outcomes. Since EVs can be stored for future use with relative ease and ability to be generated at small scales, EV-based regenerative therapeutics are further advantageous.
The potential implications of combined EV and biomaterial therapeutics allow for a tailored, predictable, tissue/patientspecific approach to regeneration, which is highly desirable by both patients and clinicians. EVs and biomaterial constructs are significantly easier to manufacture, store, and regulate compared to MSCs. These attributes represent significant cost savings, as well as increased likelihood of clinical adoption as these technologies would not require sophisticated technical expertise or equipment to implement into existing clinical workflows. As a result of the increased bio-instructive nature of optimized EV-biomaterial platforms, we believe that this may lead to simpler cell sourcing. EVs have been demonstrated to induce cell migration both in vitro and in vivo, sufficient to catalyze wound healing without requiring the transplantation of exogenous cells (Swanson et al., 2020b; Swanson et al., 2020c). In the same way, when exogenous cells are necessary, significant ex vivo autologous cell preparation (i.e., flow cytometry, ex vivo expansion) may be minimized as an instructive combination of EVs and biomaterial matrix provide sufficient selection criteria for regenerative cell populations, allowing more crude preparations.
Conclusion
Predictability of regenerative outcomes is ultimate goal of next generation tissue engineering technology. In this Viewpoint, we highlight the synergy for development of biomaterial platforms which contain EVs, rather than rely on transplantation of stem cells. EVs in conjunction with tuned biomaterials matrices represent an exciting avenue for discovery, translation, and commercialization. We believe that EV-based biomaterial technologies hold the potential to democratize access to regenerative medicine therapeutics across medical disciplines and care settings given their decreased cost, increased manufacturing throughput, advantageous storage character and potentially easier point of care use. Successful clinical translation of these technologies will continue to rely on an intimate understanding of the molecular cargo encapsulated by EVs, interactions at cell-biomaterial interface and means of efficient EV delivery. We believe that regenerative potential represents a significant benefit to patients for a variety of conditions; therapeutic approaches which circumvent challenges associated with, such as EV-based therapies, will allow for more expedient clinical trials, regulatory approval, and widespread clinical adoption, ultimately improving patient care outcomes and quality of life.
Acknowledgement:
The authors acknowledge and thank our collaborators, Prof. Nan Hatch, Prof. Peter Ma, and Dr. Maiko Omi for their intellectual contribution to our mutual work.
Funding Statement:
This work was supported by the National Institutes of Health (NIH): R01-DE027662 (YM), F30-DE029359 (WBS).
Footnotes
Ethical Approval: Mice were maintained and used in compliance with the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan in accordance with the National Institutes of Health Guidelines for Care and Use of Animals in research, and all experimental procedures were approved by the IACUC of the University of Michigan (protocol#: PRO00009613).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present work.
Availability of Data and Materials:
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- Agmon G, Christman KL (2016). Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Current Opinion in Solid State & Materials Science 20: 193–201. DOI 10.1016/j.cossms.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuma P, Okagu OD, Udenigwe CC (2019). Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Frontiers in Sustainable Food Systems 3: 341. DOI 10.3389/fsufs.2019.00023. [DOI] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology 29: 341–345. DOI 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- An Y, Zhao J, Nie F, Wu Y, Xia Y, Li D (2019). Parathyroid hormone (PTH) promotes ADSC osteogenesis by regulating SIK2 and Wnt4. Biochemical and Biophysical Research Communications 516: 551–557. DOI 10.1016/j.bbrc.2019.06.084. [DOI] [PubMed] [Google Scholar]
- Bahr MM, Amer MS, Abo-El-Sooud K, Abdallah AN, El-Tookhy OS (2020). Preservation techniques of stem cells extracellular vesicles: A gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. International Journal of Veterinary Science and Medicine 8: 1–8. DOI 10.1080/23144599.2019.1704992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuls RG, Jiya TU, Smit TH (2008). Scaffold stiffness influences cell behavior: Opportunities for skeletal tissue engineering. The Open Orthopaedics Journal 2: 103–109. DOI 10.2174/1874325000802010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenviriyakul C, Takahashi Y, Nishikawa M, Takakura Y (2018). Preservation of exosomes at room temperature using lyophilization. International Journal of Pharmaceutics 553: 1–7. DOI 10.1016/j.ijpharm.2018.10.032. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xue K, Zhang X, Zheng Z, Liu K (2018). Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Research & Therapy 9: 9406. DOI 10.1186/s13287-018-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching RC, Kingham PJ (2015). The role of exosomes in peripheral nerve regeneration. Neural Regeneration Research 10: 743–747. DOI 10.4103/1673-5374.156968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao IL, Corteling R, Bracewell D, Wall I (2018). Manufacturing exosomes: A promising therapeutic platform. Trends in Molecular Medicine 24: 242–256. DOI 10.1016/j.molmed.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Cui Y, Luan J, Li H, Zhou X, Han J (2016). Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Letters 590: 185–192. DOI 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- Dai J, Escara-Wilke J, Keller JM, Jung Y, Taichman RS, Pienta KJ, Keller ET (2019). Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. Journal of Experimental Medicine 216: 2883–2899. DOI 10.1084/jem.20190158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S, Zeh N, Schneider H, Mathias S, Raab N et al. (2019). Human CAP cells represent a novel source for functional, miRNA-loaded exosome production. PLoS One 14: e0221679. DOI 10.1371/journal.pone.0221679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano TJ, Vaso K, Danias G, Chionuma HN, Weiser JR, Iatridis JC (2021). Extracellular vesicles as an emerging treatment option for intervertebral disc degeneration: Therapeutic potential, translational pathways, and regulatory considerations. Advanced Healthcare Materials 11: 2100596. DOI 10.1002/adhm.202100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M, Zhang Y, Zhang H, Meng Y, Qian M, Zhang G (2020). Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Research & Therapy 11: 75. DOI 10.1186/s13287-020-01971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Baradie KBY, Nouh M, O’Brien III F, Liu Y, Fulzele S, Eroglu A, Hamrick MW (2020). Freeze-dried extracellular vesicles from adipose-derived stem cells prevent hypoxia-induced muscle cell injury. Frontiers in Cell and Developmental Biology 8: 301. DOI 10.3389/fcell.2020.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B, Raposo G (2004). Exosomes: Endosomal-derived vesicles shipping extracellular messages. Current Opinion in Cell Biology 16: 415–421. DOI 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and Tissue Kinetics 3: 393–403. DOI 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Garaeva L, Kamyshinsky R, Kil Y, Varfolomeeva E, Verlov N et al. (2021). Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Scientific Reports 11: 347. DOI 10.1038/s41598-021-85833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg-Worisek AJ, Runge BK, Solyntjes SA, St. Helene-Kraft J, Glass SL et al. (2018). Establishing a current good manufacturing practice facility for biomaterials and biomolecules in an academic medical center. Tissue Engineering Part B: Reviews 24: 493–498. DOI 10.1089/ten.teb.2018.0114. [DOI] [PubMed] [Google Scholar]
- Gupte MJ, Swanson WB, Hu J, Jin X, Ma H et al. (2018). Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomaterialia 82: 1–11. DOI 10.1016/j.actbio.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha DH, Kim HK, Lee J, Kwon HH, Park GH et al. (2020). Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells 9: 1157. DOI 10.3390/cells9051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harn HJ, Chen YS, Lin EY, Chiou TW (2020). Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Medical Journal 32: 113. DOI 10.4103/tcmj.tcmj_182_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman T, Khademhosseini A, Langer R (2019). Chasing the paradigm: Clinical translation of 25 years of tissue engineering. Tissue Engineering Part A 25: 679–687. DOI 10.1089/ten.tea.2019.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu GW, Li Q, Niu X, Hu B, Liu J et al. (2015). Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Research & Therapy 6: 561. DOI 10.1186/scrt546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J, Rody WJ Jr., McHugh KP, Holliday LS (2016). Characterization of regulatory extracellular vesicles from osteoclasts. Journal of Dental Research 95: 673–679. DOI 10.1177/0022034516633189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AG, Cheng K, Marban E (2014). Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2: 606–619. DOI 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari D, Shajari S, Jafari R, Mardi N, Gomari H, Ganji F, Forouzandeh Moghadam M, Samadikuchaksaraei A (2020). Designer exosomes: A new platform for biotechnology therapeutics. BioDrugs 34: 567–586. DOI 10.1007/s40259-020-00434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HK, Kim BS (2010). Modulation of stem cell differentiation with biomaterials. International Journal of Stem Cells 3: 80–84. DOI 10.15283/ijsc.2010.3.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P, Lim R, Ng J, Vemuri MC (2021). Acceleration of translational mesenchymal stromal cell therapy through consistent quality GMP manufacturing. Frontiers in Cell and Developmental Biology 9: 338. DOI 10.3389/fcell.2021.648472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat M, Bobkov I, Kumar S, Grumet M (2019). Trends in mesenchymal stem cell clinical trials 2004–2018: Is efficacy optimal in a narrow dose range? Stem Cells Translational Medicine 9: 17–27. DOI 10.1002/sctm.19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R (2016). A decade of progress in tissue engineering. Nature Protocols 11: 1775–1781. DOI 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- Kim J, Song Y, Park CH, Choi C (2021). Platform technologies and human cell lines for the production of therapeutic exosomes. Extracellular Vesicles and Circulating Nucleic Acids 2: 3–17. DOI 10.20517/evcna.2020.01. [DOI] [Google Scholar]
- Kitami M, Kaku M, Rocabado JMR, Ida T, Akiba N, Uoshima K (2016). Prolonged survival of transplanted osteoblastic cells does not directly accelerate the healing of calvarial bone defects. Journal of Cellular Physiology 231: 1974–1982. DOI 10.1002/jcp.25302. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti J (2016). Advances in tissue engineering. Journal of Pediatric Surgery 51: 8–12. DOI 10.1016/j.jpedsurg.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Vacanti JP (1993). Tissue engineering. Science 260: 920–926. DOI 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Leach JK, Whitehead J (2018). Materials-directed differentiation of mesenchymal stem cells for tissue engineering and regeneration. ACS Biomaterials Science & Engineering 4: 1115–1127. DOI 10.1021/acsbiomaterials.6b00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126: 2601–2611. DOI 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yoon JY, Lee JH, Lee HH, Knowles JC, Kim HW (2021). Emerging biogenesis technologies of extracellular vesicles for tissue regenerative therapeutics. Journal of Tissue Engineering 12: 204173142110190. DOI 10.1177/20417314211019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenas P (2018). Developmental biology in bioartificial tissue design: Manufacturing and regulatory considerations. Regenerative Medicine 13: 7–11. DOI 10.2217/rme-2017-0126. [DOI] [PubMed] [Google Scholar]
- Loh QL, Choong C (2013). Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Engineering Part B: Reviews 19: 485–502. DOI 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Brunet MY, Jones MC, Cox SC (2020). Engineered extracellular vesicles: Tailored-made nanomaterials for medical applications. Nanomaterials 10: 1838. DOI 10.3390/nano10091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC et al. (2018). Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3: 1122. DOI 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadasi S, Elveny M, Rahman HS, Suksatan W, Jalil AT et al. (2021). A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. Journal of Translational Medicine 19: 215. DOI 10.1186/s12967-021-02980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad G, Xu J, Bulte JWM, Jablonska A, Walczak P, Janowski M (2017). Transplanted adipose-derived stem cells can be short-lived yet accelerate healing of acid-burn skin wounds: A multimodal imaging study. Scientific Reports 7: 545. DOI 10.1038/s41598-017-04484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Huang CC, Ravindran S (2016). Hijacking the cellular mail: Exosome mediated differentiation of mesenchymal stem cells. Stem Cells International 2016: 1–11. DOI 10.1155/2016/3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Galipeau J (2019). Manufacturing mesenchymal stromal cells for clinical applications: A survey of good manufacturing practices at U.S. academic centers. Cytotherapy 21: 782–792. DOI 10.1016/j.jcyt.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Pinho AG, Cibrao JR, Silva NA, Monteiro S, Salgado AJ (2020). Cell secretome: Basic insights and therapeutic opportunities for CNS Disorders. Pharmaceuticals 13: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesenberry PJ, Aliotta JM (2010). Cellular phenotype switching and microvesicles. Advanced Drug Delivery Reviews 62: 1141–1148. DOI 10.1016/j.addr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani S, Ryan AE, Griffin MD, Ritter T (2015). Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Molecular Therapy 23: 812–823. DOI 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riau AK, Ong HS, Yam GHF, Mehta JS (2019). Sustained delivery system for stem cell-derived exosomes. Frontiers in Pharmacology 10: 1368. DOI 10.3389/fphar.2019.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha S, Carvalho J, Oliveira P, Voglstaetter M, Schvartz D et al. (2019). 3D cellular architecture affects microRNA and protein cargo of extracellular vesicles. Advanced Science 6: 1800948. DOI 10.1002/advs.201800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Sharma S, Korutla L, Datla SR, Shoja-Taheri F et al. (2019). Circulating exosomes derived from transplanted progenitor cells aid the functional recovery of ischemic myocardium. Science Translational Medicine 11: 201. DOI 10.1126/scitranslmed.aau1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Nogués C, O’Brien T (2021). Current good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomaterials and Biosystems 2: 100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C (2012). Gintuit cell therapy approval signals shift at US regulator. Nature Biotechnology 30: 479. DOI 10.1038/nbt0612-479. [DOI] [PubMed] [Google Scholar]
- Sensebé L, Gadelorge M, Fleury-Cappellesso S (2013). Production of mesenchymal stromal/stem cells according to good manufacturing practices: A review. Stem Cell Research & Therapy 4: 267. DOI 10.1186/scrt217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Xu J, Guo X (2017). GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Frontiers in Physiology 8: 904. DOI 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Liu X, Hu J, Wang P, Ma PX (2009). Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Engineering Part A 15: 1855–1864. DOI 10.1089/ten.tea.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squillaro T, Peluso G, Galderisi U (2016). Clinical trials with mesenchymal stem cells: An update. Cell Transplantation 25: 829–848. DOI 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- Swanson WB, Gong T, Zhang Z, Eberle M, Niemann D, Dong R, Rambhia KJ, Ma PX (2020a). Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. Journal of Controlled Release 324: 679–694. DOI 10.1016/j.jconrel.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WB, Gong T, Zhang Z, Eberle M, Niemann D, Dong R, Rambhia KJ, Ma PX (2020b). Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. Journal of Controlled Release 324: 679–694. DOI 10.1016/j.jconrel.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WB, Ma PX (2020). Nanofibrous and porous biomaterials. In: Zhang G (ed.), Biomaterials Science, An Introduction to Materials in Medicine. Elsevier. https://www.sciencedirect.com/science/article/pii/B9780128161371000398. [Google Scholar]
- Swanson WB, Omi M, Zhang Z, Nam HK, Jung Y, Wang G, Ma PX, Hatch NE, Mishina Y (2021). Macropore design of tissue engineering scaffolds regulates mesenchymal stem cell differentiation fate. Biomaterials 272: 120769. DOI 10.1016/j.biomaterials.2021.120769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WB, Zhang Z, Xiu K, Gong T, Eberle M, Wang Z, Ma PX (2020c). Scaffolds with controlled release of promineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomaterialia 118: 215–232. DOI 10.1016/j.actbio.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SSH, Tjio CKE, Wong JRY, Wong KL, Chew JRJ, Hui JHP, Toh WS (2021). Mesenchymal stem cell exosomes for cartilage regeneration: A systematic review of preclinical in vivo studies. Tissue Engineering Part B: Reviews 27: 1–13. DOI 10.1089/ten.teb.2019.0326. [DOI] [PubMed] [Google Scholar]
- ten Ham RMT, Hövels AM, Hoekman J, Frederix GWJ, Leufkens HGM et al. (2020). What does cell therapy manufacturing cost? A framework and methodology to facilitate academic and other small-scale cell therapy manufacturing costings. Cytotherapy 22: 388–397. DOI 10.1016/j.jcyt.2020.03.432. [DOI] [PubMed] [Google Scholar]
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 7: 1535750. DOI 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology 19: 213–228. DOI 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Williams DF (2019). Challenges with the development of biomaterials for sustainable tissue engineering. Frontiers in Bioengineering and Biotechnology 7: 155. DOI 10.3389/fbioe.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, van Balkom BWM, Bruno S, Choo A, Dominici M et al. (2019). Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. Journal of Extracellular Vesicles 8: 1609206. DOI 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M et al. (2015). Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. International Journal of Cardiology 178: 239–246. DOI 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- Yingst DR, Hoffman JF (1984). Ca-induced K transport in human red blood cell ghosts containing arsenazo III. Transmembrane interactions of Na, K, and Ca and the relationship to the functioning Na-K pump. Journal of General Physiology 83: 19–45. DOI 10.1085/jgp.83.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y (2014). Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. Journal of Biological Chemistry 289: 24488–24498. DOI 10.1074/jbc.M114.577213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HC, Liu XB, Huang S, Bi XY, Wang HX et al. (2012). Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells and Development 21: 3289–3297. DOI 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Zhao X, Chen X, Wei Y, Du W et al. (2018a). Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Applied Materials & Interfaces 10: 30081–30091. DOI 10.1021/acsami.8b08449. [DOI] [PubMed] [Google Scholar]
- Zhang R, Ma PX (2000). Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. Journal of Biomedical Materials Research 52: 430–438. DOI 10.1002/(ISSN)1097-4636. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS (2018b). MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156: 16–27. DOI 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu H, Xu W, Wang B, Wu H et al. (2013). Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Research & Therapy 4: 34. DOI 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z et al. (2017). Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. Journal of Extracellular Vesicles 6: 1324730. DOI 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zhang L, Yang L, Zhu F, Ding M, Lin F, Wang Z, Li Y (2018). Click chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. Journal of Controlled Release 273: 160–179. DOI 10.1016/j.jconrel.2018.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


