Abstract
Patients at high risk for sudden cardiac death (SCD) may benefit from wearable cardioverter defibrillators (WCD) by avoiding immediate implantable cardioverter defibrillator (ICD) implantation. Different factors play an important role including patient selection, compliance and optimal drug treatment. We aimed to present real world data from 4 centers from Germany and Switzerland. Between 04/2012 and 03/2019, 708 patients were included in this registry. Patients were followed up over a mean time of 28 ± 35.5 months. Outcome data including gender differences and different etiologies of cardiomyopathy were analyzed. Out of 708 patients (81.8% males, mean age 61.0 ± 14.6), 44.6% of patients had non-ischemic cardiomyopathy, 39.8% ischemic cardiomyopathy, 7.9% myocarditis, 5.4% prior need for ICD explantation and 2.1% channelopathy. The mean wear time of WCD was 21.2 ± 4.3 h per day. In 46% of patients, left ventricular ejection fraction (LVEF) was > 35% during follow-up. The younger the patient was, the higher the LVEF and the lower the wear hours per day were. The total shock rate during follow-up was 2.7%. Whereas an appropriate WCD shock was documented in 16 patients (2.2%), 3 patients received an inappropriate ICD shock (0.5%). During follow-up, implantation of a cardiac implantable electronic device was carried out in 34.5% of patients. When comparing German patients (n = 516) to Swiss patients (n = 192), Swiss patients presented with longer wear days (70.72 ± 49.47 days versus 58.06 ± 40.45 days; p = 0.001) and a higher ICD implantation rate compared to German patients (48.4% versus 29.3%; p = 0.001), although LVEF at follow-up was similar between both groups. Young age is a negative independent predictor for the compliance in this large registry. The most common indication for WCD was non-ischemic cardiomyopathy followed by ischemic cardiomyopathy. The compliance rate was generally high with a decrease of wear hours per day at younger age. Slight differences were found between Swiss and German patients, which might be related to differences in mentality for ICD implantation.
Subject terms: Ultrasonography, Ventricular fibrillation, Ventricular tachycardia
Introduction
Sudden cardiac death (SCD) can potentially be prevented using the wearable cardioverter defibrillator (WCD). A bevy of indications have been highlighted for the use of WCD e.g. for secondary or primary prevention of patients with ischemic (ICM) or non-ischemic cardiomyopathies (NICM) or in patients after removal of infected implantable cardioverter defibrillators (ICDs)1 or in patients post myocarditis, peripartum cardiomyopathy and takotsubo syndrome26–29.
One of the main parameters for risk stratification for SCD is the left ventricular ejection fraction (LVEF ≤ 35%)2,3. Up to date, data have shown that patients with a severely reduced LVEF and heart failure are at high risk of SCD4–6. Several data sets have shown that patients with newly diagnosed heart failure caused by ICM or NICM may present with an improvement of LVEF subsequent to optimal medical treatment (OMT). Therefore, current guidelines support a strategy of waiting for at least 6 weeks to 3 months after the initial diagnosis of highly reduced LVEF depending on the underlying etiology7,8. Data of the IRIS and DINAMIT trials showed that early ICD implantation after MI does not improve the survival rates9,10. Moreover, recently published data debated a prolonged period of OMT and a prolonged WCD use before ICD implantation in patients with newly diagnosed NICM and ICM30. Prolonged WCD use and OMT may allow avoiding over-use of ICDs13.
Although ICD implantation is feasible in young patients, a multitude of device-related complications including inappropriate shocks, infections, lead failure and lead dysfunction, which increase over time, have been reported, which may potentially be avoided by unnecessary ICD implantation.
Recently published data of the VEST trial14 showed that WCD do not significantly impact the outcome, measured by a composite endpoint after up to 90 days of use in patients with MI and moderate to severe left ventricular dysfunction compared to controls. Although a significant reduction in all-cause mortality (secondary endpoint), a safe use of WCD and a very low rate of inappropriate shocks in the WCD group were documented, the published data does not convincingly support the systematic use of WCD in this population.
In the present study, we investigated 708 consecutive patients from four hospitals in Germany and Switzerland. Long-term follow-up data over a mean time of 2.5 years was collected. We aimed to investigate the risk of ventricular tachyarrhythmias and the success rate of termination of these by WCD use.
Methods
Patient recruitment
We included 708 patients at four hospitals (University Mannheim, Frankfurt University Hospital, the Heart Center Leipzig and the University Hospital Zurich). Patients were included between 04/2012 and 03/2019. All patients received OMT. Patients were fitted with a ZOLL Life Vest System. The study was approved by the local ethics committee (2019-840-R and BASEC 2017-01044) and conforms to the 1975 Declaration of Helsinki. An informed consent was waived by the ethics committee to the retrospective character of the study. All methods were performed in accordance with the relevant guidelines and regulations by including a statement in the “Methods” section.
The wearable cardioverter-defibrillator (WCD)
The WCD ZOLL Life Vest ™system (Pittsburgh, USA) and programmed data have been recently described in depth15. Different points were taken into consideration for programming including the underlying heart disease and electrocardiographic patterns. In general, for older patients the ventricular tachycardia (VT) zone was programmed at a heart rate of 150–190 bpm with a VT response time of 60 s and for younger patients a VT zone was programmed at a heart rate of 180–190 bpm with a VT response time also of 60 s. The ventricular fibrillation (VF) zone was programmed similarly in older and younger patients at a heart rate of 200–220 bpm with a response time of 25 s. The maximum first shock energy was 150 J with a separate episode detecting when episodes were common with a minimum delay of 3 min. Episodes were reviewed and classified by independent physicians. Episodes were separated into one of two groups, sustained VT (lasting 30 s or longer) or VF with WCD shock therapy and non-sustained VT (lasting less than 30 s) without WCD shock. Inappropriate WCD therapy was identified as non-ventricular tachyarrhythmias or non-ventricular fibrillation episode treated by an inappropriate WCD shock.
Baseline and follow up data collection
Baseline characteristics were evaluated at each center including the indication for WCD use, the disease etiology, the initial LVEF calculated by the biplane Simpson’s method using echocardiography and/or cardiac magnetic resonance imaging (MRI). LVEF improvement was defined as an increase of the LVEF > 35% over follow-up. The optimal medical treatment (OMT) was documented in 96% of patients in the cohort. However, the dosage of used drugs was not studied systematically. In general, WCD use was suggested for 3 months regardless of the underlying heart disease. WCDs were prescribed consistent with current guidelines and the risk was estimated and individualized by treating physicians. Data on arrhythmias during follow-up were prospectively collected clinically and retrieved from the ZOLL Life Vest Network™.
After prescription WCD, patients were followed for a mean follow-up of 28 ± 35.5 months. This mean follow-up included follow-up after ICD-implantation. To estimate the need of ICD implantation, the LVEF was evaluated 3–6 months after the initial evaluation. If the LVEF was > 35% and no ventricular tachyarrhythmias were detected no ICD was implanted concomitant with current guidelines. In some cases of a relevant improvement of LVEF but nevertheless LVEF < 36%, prolongation of WCD wearing was applied. This was dependent on physician–patient decision.
OMT was achieved using the generally recommended heart failure drugs e.g. angiotensin converting-enzyme inhibitor/angiotensin receptor blocker (ACE-I/ARB), beta-blockers and mineralocorticoid receptor blocker (MRA) consistent with current heart failure guidelines3. Also, angiotensin receptor-neprilysin inhibitors (ARNI) were used instead of ACE-I or ARB consistent with published data and guidelines31-36.
Patient charts, general physicians, cardiologists, patients and family members were consulted for updating missing data.
Statistics
Data are presented as mean ± standard deviation for continuous variables or as frequencies and percentages for categorical variables. For the comparison of continuous variables the paired-t-test was used and for the comparison of categorical variables a chi-squared test or Fisher's exact test was used.
A two-sided P value < 0.05 was considered statistically significant. IBM SPSS for Macintosh (Version 25.0. Armonk, NY: IBM Corp.) was used for statistical analyses. For predictor analysis Cox logistic regression analysis was done. Factors with a p value < 0.1 were included in a multivariable logistic regression analysis.
Results
Patients’ baseline characteristics
The indications for WCD use were predominantly ICM and NICM (39.9% and 44.6%). Other indications were myocarditis, ICD explantation and, in rare cases, channelopathy (Fig. 1). The reason for prescription WCD in ICM, NICM and myocarditis patients was the low LVEF. 81.8% of patients were male and 18.2% were female. The shock rate was 2.7% (n = 19), appropriate in 2.2% and inappropriate in 0.5% of patients. The mean wear time of WCD per day was 21.17 ± 4.31 h and mean wear day time was 61 days. The index LVEF was 29.66 ± 11.36% and the follow-up LVEF was 37.5 ± 12.35%. In 42.9% of patients an improvement of the LVEF was documented, with 46% of patients having a LVEF > 35% at follow-up. Implantation of a cardiac implantable electronic device was carried out in 47.7% of patients (Table 1). The satisfactory compliance rate, defined as wear hours > 20 h per day, of the whole cohort was 78.4%. The overall death rate in the cohort was 5.9%. No ventricular tachyarrhythmias were documented once the WCD was no more in use and the LVEF was improved > 35% in these patients, who did not receive an ICD.
Figure 1.
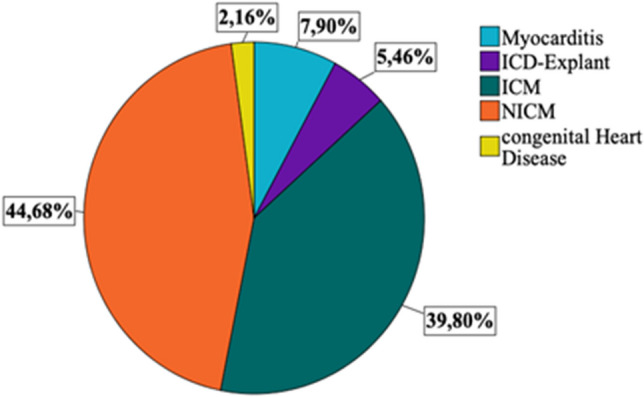
The distribution of indication for WCD us in the general cohort.
Table 1.
Baseline characteristics of 708 patients with WCD use.
| Variables | Patients (n = 708) |
|---|---|
| Female, n (%) | 129 (18.2) |
| Age, mean ± SD | 60.68 ± 14.63 |
| WCD wear time (h/day), mean ± SD | 21.16 ± 4.34 |
| WCD wear days, mean ± SD | 61.5 ± 43.45 |
| WCD shocks, n (%) | 19 (2.7) |
| Appropriate | 16 (2.2) |
| Inappropriate | 3 (0.5) |
| EF baseline, mean ± SD | 29.62 ± 11.20 |
| EF follow-up, mean ± SD | 37.27 ± 12.26 |
| EF improvement, n (%) | 326 (46.0) |
| Device implantation, n (%) | 244 (34.5) |
| Death during follow-up, n (%) | 42 (5.9) |
| Compliance (> 20 h/day WCD use), n (%) | 555 (78.4) |
WCD data stratified by sex
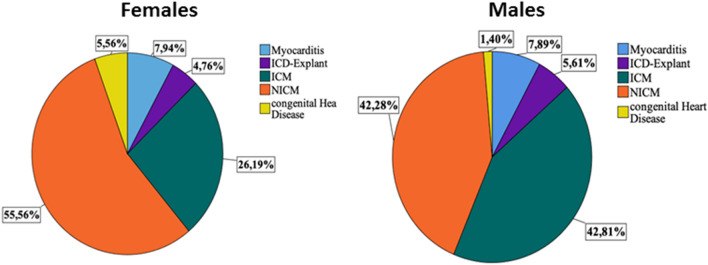
The mean average age and wear days were comparable in females and males at the time of WCD prescription (Table 2). The appropriate WCD shock rate was also comparable between females and males (3.8% versus 1.9%; p = 0.259). In males, the WCD use was similar in ICM and NICM. On the other hand, NICM was predominantly the cause for WCD use in females, Fig. 2. The death rate was comparable between males and females.
Table 2.
Baseline characteristics of WCD patients stratified by sex.
| Female, n = 129 | Male, n = 579 | p-Value | |
|---|---|---|---|
| Age, mean ± SD | 61.58 ± 17.05 | 60.48 ± 14.04 | 0.441 |
| WCD wear time (h/day), mean ± SD | 21.53 ± 4.41 | 21.09 ± 4.29 | 0.282 |
| WCD wear days, mean ± SD | 56.60 ± 38.98 | 62.86 ± 44.36 | 0.140 |
| WCD shocks, n (%) | 5 (3.8) | 14 (2.4) | 0.082 |
| Appropriate | 5 (3.8) | 11 (1.9) | 0.259 |
| Inappropriate | 0 (0) | 3 (0.5) | 0.259 |
| EF baseline, mean ± SD | 30.17 ± 12.30 | 29.55 ± 11.14 | 0.549 |
| EF follow-up, mean ± SD | 38.18 ± 13.17 | 37.35 ± 12.17 | 0.486 |
| EF improvement > 35%, n (%) | 67 (51.9) | 259 (44.7) | 0.157 |
| Device implantation, n (%) | 41 (31.8) | 203 (35.1) | 0.330 |
| Death during follow-up, n (%) | 10 (7.7) | 32 (5.5) | 0.497 |
| Compliance (> 20 h/day WCD use), n (%) | 109 (84.5) | 446 (77.0) | 0.063 |
Figure 2.
The indication for WCD use in males and females.
WCD data stratified by etiology
Patients with ICM were significantly older compared to NICM (64.96 ± 11.09 years versus 58.62 ± 14.51 years; p = 0.001). Females were more common in the NICM group as compared to the ICM group (23.2% versus 11.7%, p = 0.001). The wear time in days and wear time in hours were comparable between both groups. The initial LVEF was significantly higher in ICM patients as compared to NICM patients, while the appropriate shock rate was similar (8 patients versus 2 patients, p = 0.371). In 44% of patients with ICM, LVEF improved during follow-up as compared to 50.8% in the NICM group. In general, all-cause mortality tended to be higher in the ICM group as compared to the NICM group (7.2% versus 4.2%, p = 0.061), (Table 3).
Table 3.
Baseline characteristics of WCD patients stratified by cardiomyopathy etiology.
| N (%) | ICM, 277 (39.7) | NICM, 315 (44.5) | p-value |
|---|---|---|---|
| Age, mean ± SD | 64.96 ± 11.11 | 58.59 ± 14.61 | 0.001 |
| Female, n (%) | 33 (11.9) | 70 (22.5) | 0.001 |
| WCD wear time (h/day), mean ± SD | 21.32 ± 3.92 | 20.92 ± 4.47 | 0.251 |
| WCD wear days, mean ± SD | 63.22 ± 43.55 | 61.66 ± 43.44 | 0.665 |
| WCD Shocks, n (%) | 9 (3.2) | 3 (1.0) | 0.026 |
| Appropriate | 8 (2.9) | 2 (0.7) | 0.371 |
| Inappropriate | 1 (0.3) | 1 (0.3) | 0.371 |
| EF baseline, mean ± SD | 30.07 ± 8.76 | 26.65 ± 10.96 | 0.001 |
| EF follow-up, mean ± SD | 36.58 ± 10.71 | 36.67 ± 12.18 | 0.920 |
| EF improvement > 35%, n (%) | 122 (44.0) | 158 (50.8) | 0.111 |
| Device implantation, n (%) | 112 (40.4) | 101 (32.5) | 0.208 |
| Death, n (%) | 20 (7.2) | 13 (4.2) | 0.061 |
| Compliance (> 20 h/day WCD use), n (%) | 219 (79.1) | 239 (76.8) | 0.754 |
Characteristics of patients treated with WCD shocks
Of the 19 patients treated with WCD shocks, 16 patients (2.2%) received an appropriate WCD shock and three patients (0.5%) inappropriate WCD shocks. Eight patients were diagnosed with ICM, two patients with NICM, three patients suffered from myocarditis and two patients received an ICD explantation after which WCD was required. The reason for a WCD in myocarditis patients was low LVEF and in one case low LVEF and non-sustained VTs.
One patient with an appropriate shock suffered from a hereditary channelopathy (Table 4). The case was a long-QT syndrome. The patient showed also a reduced LVEF at events. Since the diagnosis was not clear it was decided for a WCD prescription. Over follow-up the LVEF was normalized however the QTc was ongoing prolonged.
Table 4.
WCD Shock Characteristics according to appropriate and inappropriate manner, n = 19.
| Gender | Age | WCD Indication | LVEF at baseline | Shock appropriate | Arrhythmic episode | ICD implant | Death during 3 yr follow up | Appropriate ICD shocks |
|---|---|---|---|---|---|---|---|---|
| m | 66 | ICM | 35 | Yes | VT | Yes | No | Yes |
| f | 31 | Myocarditis | 53 | Yes | VT | Yes | No | No |
| m | 48 | ICD-Explant | 30 | Yes | VT | Yes | Yes | No |
| m | 53 | ICM | 25 | Yes | VF | Yes | No | No |
| m | 64 | ICM | 35 | Yes | VT | Yes | No | Yes |
| m | 75 | ICM | 20 | Yes | VT | Yes | No | No |
| f | 71 | ICM | 30 | Yes | VF | Yes | No | No |
| f | 84 | ICM | 25 | Yes | VT | Yes | No | No |
| m | 64 | NICM | 25 | Yes | VT | Yes | Yes | No |
| m | 71 | ICM | 27 | Yes | VF | Yes | No | Yes |
| m | 60 | NICM | 13 | Yes | VT | Yes | No | No |
| m | 64 | Myocarditis | 40 | Yes | VF | Yes | No | No |
| f | 84 | ICM | 35 | Yes | VT | No | No | No |
| m | 62 | Myocarditis | 20 | Yes | VT | Yes | No | No |
| f | 55 | ICD-Explant | 55 | Yes | VF | Yes | No | Yes |
| m | 84 | ICM | 27 | No | AFlutt | No | No | No |
| m | 25 | Channelopathy | 60 | Yes | VF | Yes | No | Yes |
| m | 78 | NICM | 30 | No | AF | Planned | Unknown | Unknown |
| m | 64 | Myocarditis | 40 | No | AF | No | No | No |
A.Flutt atrial flutter, AF atrial fibrillation, VT ventricular tachycardia.
Of the 16 patients receiving an appropriate WCD shock, 5 received appropriate ICD shocks after ICD implantation during a follow-up period of 28 months.
Characteristics of patients according to age differences
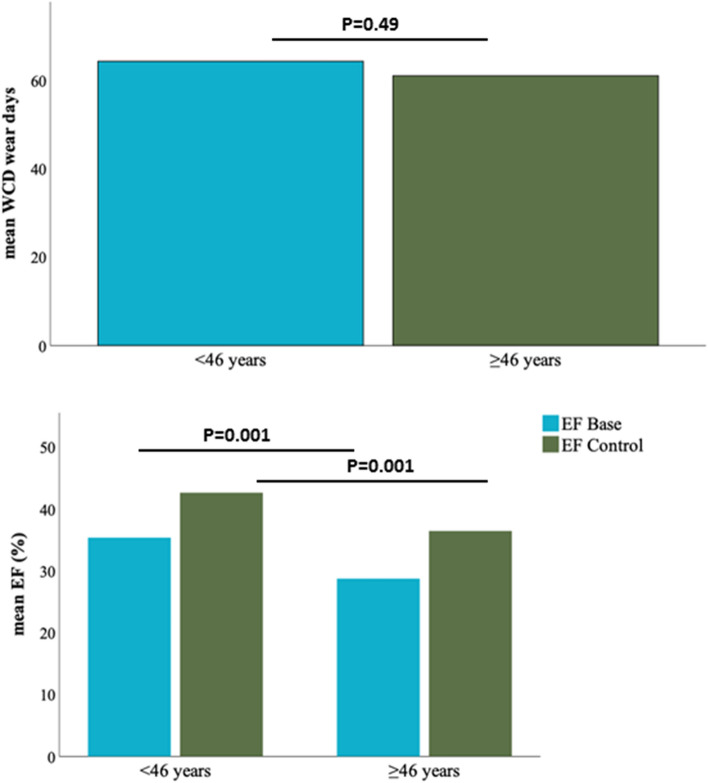
We separated patients into two groups: < 46 years, n = 94 and ≥ 46 years, n = 614. The average wear days were comparable between both cohorts. The initial LVEF, the follow up LVEF and the percentage of patients with an improvement of the LVEF was higher in the young cohort as compared to the old cohort, Fig. 3. The younger patients tended to have a lower compliance rate, however when setting a cut-off of 46 years, no significant difference in wear days was documented, Fig. S1.
Figure 3.
The comparison of WCD use according to age.
Characteristics of patients of the German and Swiss cohort
The cohort included 516 German patients and 192 Swiss patients. In the German cohort, 104 (20.34%), and in the Swiss cohort 25 (13.02%) patients were female; p = 0.02. German patients were older than Swiss patients (61.74 ± 15.103 years versus 57.84 ± 12.877, p = 0.002). Although Swiss patients presented with more wear days compared to German patients (70.72 ± 49.47 days versus 58.06 ± 40.45 days; p = 0.001), the wear hours per day was comparable in the Swiss cohort and the German cohort (21.29 ± 4.49 versus 20.79 ± 3.88, p = 0.179). The initial LVEF was significantly lower in German patients compared to Swiss patients (28.85 ± 10.38% versus 31.67 ± 12.97%), while the follow-up LVEF was comparable between both groups, Table 5, Fig. S2. On the other hand, the ICD implantation rate was significantly higher in Swiss patients compared to German patients (48.3% versus 29.3%; p = 0.001).
Table 5.
Baseline characteristics of WCD patients in Germany compared to Switzerland.
| Germany, n = 516 | Switzerland, n = 192 | p-Value | |
|---|---|---|---|
| Female, n (%) | 104 (20.34) | 25 (13.02) | 0.029 |
| Age, mean ± SD | 61.74 ± 15.10 | 57.84 ± 12.88 | 0.002 |
| WCD wear time (h/day), mean ± SD | 21.29 ± 4.49 | 20.79 ± 3.88 | 0.179 |
| WCD wear days, mean ± SD | 58.06 ± 40.45 | 70.72 ± 49.47 | 0.001 |
| WCD shocks, n (%) | 16 (3.1) | 3 (1.6) | 0.260 |
| Appropriate | 13 (2.5) | 3 (1.6) | 0.414 |
| Inappropriate | 3 (0.6) | 0 (0) | 0.414 |
| EF baseline, mean ± SD | 28.85 ± 10.38 | 31.67 ± 12.97 | 0.003 |
| EF follow-up, mean ± SD | 37.06 ± 12.49 | 37.84 ± 11.63 | 0.455 |
| EF improvement > 35%, n (%) | 241 (46.7) | 85 (44.3) | 0.563 |
| Device Implantation, n (%) | 151 (29.3) | 93 (48.4) | 0.001 |
| Compliant (> 20 h/day WCD wear time), n (%) | 413 (80.0) | 142 (74.0) | 0.081 |
Predictors of compliance
To understand, which are the predicting factors for a good compliance we did a Cox logistic regression. In the univariate analysis male gender (OR 0.61; 95%CI 0.36–1.02; p = 0.06) and age < 46 years (OR 0.60; 95%CI 0.37–0.97; p = 0.04) were predictors of the compliance. In the multivariable logistic regression analysis only young age (OR 0.58; 95%CI 0.36–0.95; p = 0.03) was a negative independent predictor of the compliance, Table 6.
Table 6.
Multivariable logistic regression analysis for the compliance.
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | |
| Male | 0.61 | 0.36–1.02 | 0.06 | 0.60 | 0.35–1.00 | 0.05 |
| Age < 46 | 0.60 | 0.37–0.97 | 0.04 | 0.58 | 0.36–0.95 | 0.03 |
| NICM | 0.85 | 0.59–1.21 | 0.37 | |||
| Myocarditis | 1.67 | 0.77–3.62 | 0.18 | |||
| ICD explantation | 0.88 | 0.40–1.90 | 0.75 | |||
| EF at baseline | 1.00 | 0.99–1.02 | 0.43 | |||
| ICM | 1.06 | 0.73–1.54 | 0.72 | |||
| WCD shocks | 5.21 | 0.70–38.48 | 0.10 | |||
| EF improvement | 1.16 | 0.80–1.66 | 0.42 | |||
OR odds ratio, EF ejection fraction, NICM non-ischemic-cardiomyopathy, WCD wearable life-vest.
Discussion
We analyzed real life experience with WCD in Germany and Switzerland. Patients were recruited in 4 centers and data was collected for three years. The findings of the present project are: (i) WCD use in the present cohort was required due to NICM followed by ICM, (ii) The daily wear time of the present cohort with 21.17 ± 4.31 h is high and implicates excellent patient compliance in Germany and Switzerland; (iii) The general shock rate is 2.7%, the appropriate shock rate being 2.2% and the inappropriate shock rate being low with 0.5%; iv) the younger the patients, the lower the compliance rate as presented by wear hours per day.
Sudden cardiac death is one of the main causes of death worldwide predominantly due to ICM16. The majority of patients suffer from ventricular tachyarrhythmias. The efficacy of WCD has been confirmed in several registries and reports15,17–19. The appropriate WCD shock rate in our study was 2.2%. This rate is slightly higher compared to other reported cohorts from the USA and France, which varied between 1.6 and 1.7%. Of the 16 patients treated with an appropriate WCD shock and subsequent ICD implantation, 5 (31%) patients suffered from an appropriate ICD shock during follow-up. Data from the WEARIT-II Registry and WCD France Registry showed that WCD is safe with low inappropriate shock rates of 0.5% and 0.7%, respectively, which was similar in our multicenter registry. In general, these data are consistent throughout multiple studies and confirm the safety of the WCD20.
Current ESC guidelines recommend that WCD may be indicated in patients with heart failure related to a decline in LVEF (≤ 35%) as a bridge solution until either recovery of LVEF, heart transplant or ICD re-implantation in case of prior device explantation or de-novo ICD implantation1. Overall, the presented data confirms the role of WCD in selected patients for the prevention of SCD. The DANISH trial, as one of the first trials focusing exclusively on patients with NICM, confirmed the risk of ventricular tachyarrhythmias in the chronic phase of NICM, during which primary preventive ICD implantation on the other hand did not reduce overall mortality21. But there was a significant interaction with age and younger patients showed a significant reduction in all-cause mortality after ICD implantation. Therefore, in NICM an individualized manner might be suggested regarding the decision on ICD implantation taking into consideration severity of underlying disease and concomitant arrhythmia risk versus age, life expectancy and risks of competing illness.
It was reported that during 12 months of follow-up, 4% of patients with WCD died and the overall death rate between ICM and NICM was comparable22. In the present cohort we found a mortality rate of 6.1% over a follow-up of almost 2.5 years and the mortality rate in the ICM group tended to be higher than the NICM group (Table 3). Data from a large cohort from the USA found a 3-month mortality rate of 3.6% and a 3-year mortality of 20.5%. This higher rate of mortality might be due to the heterogeneity of the USA cohort and different comorbidities compared to the present cohort15.
One important issue is the compliance of patients wearing the WCD. A lack of adherence to therapy (i.e. not wearing the WCD) is one of the possible causes for the lack of positive results measured in the VEST trial which included patients after acute MI14. Indeed, in a per-protocol analysis of the VEST trial the authors found a significant reduction of all-cause and arrhythmic mortality23. These results however have to be interpreted with caution due to the inherent risk of bias when performing per-protocol analyses. Nevertheless, patients should be educated and regularly reminded of the importance of wearing the WCD for the entire prescription period. In the present cohort, a high compliance rate with 78.4% of patients wearing the WCD > 20 h per day were achieved. Different reports discussed different predictors of this adherence to wear the WCD. Recently published data reported that a younger age is associated with a less compliance20. Our data shows a different compliance rate for wearing the WCD from 16 to 46 years old. After age of 45 years no significant difference regarding the compliance was documented. We evaluated predictors of the compliance and found young age is a negative independent predictor of the compliance. This is might be related to the character of young patients with underestimating their disease and/or absence of symptoms.
Data from other reports showed that the average hours of use per day were 20.7 in males and 21.4 in females (p = 0.001) with a repetitive WCD shock rate in females suffering from ICM24,25. In the present cohort we found similar average wear hours in females compared to males. In general, our data present a high compliance for WCD use consistent with other reports.
Beside the VEST trial which had a low daily compliance of 14 ± 9.3 h, other reports, albeit from registry data, present a high compliance of patients. Therefore, different factors should be taken into consideration when WCD is prescribed such as sufficient education and training of the patient. Additionally, close follow-ups are required to avoid failure rates regarding to incompliance.
Limitations of the study
The present study has several limitations. Firstly, the use of magnetic resonance imaging for the outcome of patients and risk stratification of WCD cohort is not considered. In addition, the prescription was based on physician decision and individualized risk factors. Thirdly, a detailed cause of death was not evaluated in a systematic manner in patients. Finally, a further evaluation of predictors for appropriate WCD shock rate according to the cause of NICM form is not possible related to the low WCD shock rate, which should be analyzed in an expanded cohort.
Conclusion
The most common indication for WCD was NICM followed by ICM. The compliance rate was generally high with a decrease of wear hours per day at younger age. Slight differences were found between Swiss and German patients, which might be related to differences in mentality for ICD implantation.
Supplementary Information
Author contributions
I.E.-B., T.D., B.K. and I.A. wrote the paper. J.K.: checked the language. T.D., B.K., J.E., N.K., S.R., A.S., and F.D.: analysed the data. F.D., I.A., J.K., S.R. and S.R. did a critical review. T.D. and I.E.-B.did the statistical analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06007-y.
References
- 1.Priori SG, Blomstrom-Lundqvist C. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur. Heart J. 2015;36:2757–2759. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur. Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev. Esp. Cardiol. (Engl. Ed.) 2016;69(1167):2016. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Adabag AS, Therneau TM, Gersh BJ, Weston SA, Roger VL. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns RJ, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J. Am. Coll. Cardiol. 2002;39:30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 6.Kadish A, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 7.Priori SG, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Kardiol. Pol. 2015;73:795–900. doi: 10.5603/KP.2015.0190. [DOI] [PubMed] [Google Scholar]
- 8.Solomon SD, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 9.Steinbeck G, et al. Defibrillator implantation early after myocardial infarction. N. Engl. J. Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser SH, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N. Engl. J. Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 11.Duncker D, Konig T, Hohmann S, Bauersachs J, Veltmann C. Avoiding untimely implantable cardioverter/defibrillator implantation by intensified heart failure therapy optimization supported by the wearable cardioverter/defibrillator: The PROLONG study. J. Am. Heart Assoc. 2017;6:4512. doi: 10.1161/JAHA.116.004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncker D, Konig T, Hohmann S, Bauersachs J, Veltmann C. Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study. Clin. Cardiol. 2017;40:586–590. doi: 10.1002/clc.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreher TC, et al. Comparison of the outcome of patients protected by the wearable cardioverter defibrillator (WCD) for <90 wear days versus >/=90 wear days. In Vivo. 2020;34:3601–3610. doi: 10.21873/invivo.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olgin JE, et al. Wearable cardioverter-defibrillator after myocardial infarction. N. Engl. J. Med. 2018;379:1205–1215. doi: 10.1056/NEJMoa1800781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung MK, et al. Aggregate national experience with the wearable cardioverter-defibrillator: Event rates, compliance, and survival. J. Am. Coll. Cardiol. 2010;56:194–203. doi: 10.1016/j.jacc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 17.Kutyifa V, et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry) Circulation. 2015;132:1613–1619. doi: 10.1161/CIRCULATIONAHA.115.015677. [DOI] [PubMed] [Google Scholar]
- 18.Wassnig NK, et al. Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation. 2016;134:635–643. doi: 10.1161/CIRCULATIONAHA.115.019124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman AM, et al. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: Results of the WEARIT/BIROAD. Pacing Clin. Electrophysiol. 2004;27:4–9. doi: 10.1111/j.1540-8159.2004.00378.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia R, et al. Wearable cardioverter-defibrillator in patients with a transient risk of Sudden cardiac death: The WEARIT-France cohort study. Europace. 2020 doi: 10.1093/europace/euaa268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kober L, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N. Engl. J. Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 22.Kutyifa V, et al. One-year follow-up of the prospective registry of patients using the wearable defibrillator (WEARIT-II Registry) Pacing Clin. Electrophysiol. 2018;41:1307–1313. doi: 10.1111/pace.13448. [DOI] [PubMed] [Google Scholar]
- 23.Olgin JE, et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J. Cardiovasc. Electrophysiol. 2020;31:1009–1018. doi: 10.1111/jce.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg I, et al. Sex-differences in arrhythmic burden with the wearable cardioverter defibrillator. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Erath JW, et al. Sustained ventricular tachyarrhythmia termination in a large cohort of women using wearable cardioverter-defibrillators. JACC Clin. Electrophysiol. 2020;6:1187–1188. doi: 10.1016/j.jacep.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Rosenkaimer SL, El-Battrawy I, Dreher TC, Gerhards S, Röger S, Kuschyk J, Borggrefe M, Akin I. The wearable cardioverter-defibrillator: Experience in 153 patients and a long-term follow-up. J Clin Med. 2020;9(3):893. doi: 10.3390/jcm9030893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Battrawy, I. et al. Takotsubo syndrome and cardiac implantable electronic device therapy. Sci Rep.9(1), 16559. 10.1038/s41598-019-52929-5 (2019). [DOI] [PMC free article] [PubMed]
- 28.El-Battrawy, I. et al. Implantable cardioverter-defibrillator in Brugada syndrome: Long-term follow-up. Clin Cardiol.42(10), 958–965. 10.1002/clc.23247 (2019). [DOI] [PMC free article] [PubMed]
- 29.El-Battrawy, I. et al. Long-term follow-up of implantable cardioverter-defibrillators in Short QT syndrome. Clin Res Cardiol.108(10), 1140–1146. 10.1007/s00392-019-01449-3 (2019). [DOI] [PubMed]
- 30.Dreher, T. C. et al. Comparison of the outcome of patients protected by the wearable cardioverter defibrillator (WCD) for <90 wear days versus ≥90 wear days. In Vivo.34(6), 3601–3610. 10.21873/invivo.12205 (2020). [DOI] [PMC free article] [PubMed]
- 31.Abumayyaleh, M., El-Battrawy, I., Behnes, M., Borggrefe, M. & Akin, I. Current evidence of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Future Cardiol.16(4), 227–236. 10.2217/fca-2020-0002 (2020). [DOI] [PubMed]
- 32.Aktaa, S. et al. European Society of Cardiology quality indicators for the care and outcomes of adults with heart failure. Developed by the Working Group for Heart Failure Quality Indicators in collaboration with the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail.24(1), 132–142. 10.1002/ejhf.2371 (2022). [DOI] [PubMed]
- 33.El-Battrawy, I. et al. Impact of Sacubitril/Valsartan on the Long-Term Incidence of Ventricular Arrhythmias in Chronic Heart Failure Patients. J Clin Med.8(10), 1582. 10.3390/jcm8101582 (2019). [DOI] [PMC free article] [PubMed]
- 34.El-Battrawy, I., Borggrefe, M. & Akin, I. The Risk for Sudden Cardiac Death and Effect of Treatment With Sacubitril/Valsartan in Heart Failure. JACC Heart Fail.7(11), 999 (2019). [DOI] [PubMed]
- 35.Abumayyaleh, M. et al. Comparison of the prognosis and outcome of heart failure with reduced ejection fraction patients treated with sacubitril/valsartan according to age. Future Cardiol.17(6), 1131–1142. 10.2217/fca-2020-0213 (2021). [DOI] [PubMed]
- 36.Abumayyaleh, M. et al. Clinical outcomes in patients with ischemic versus non-ischemic cardiomyopathy after angiotensin-neprilysin inhibition therapy. J Clin Med.10(21), 4989 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.