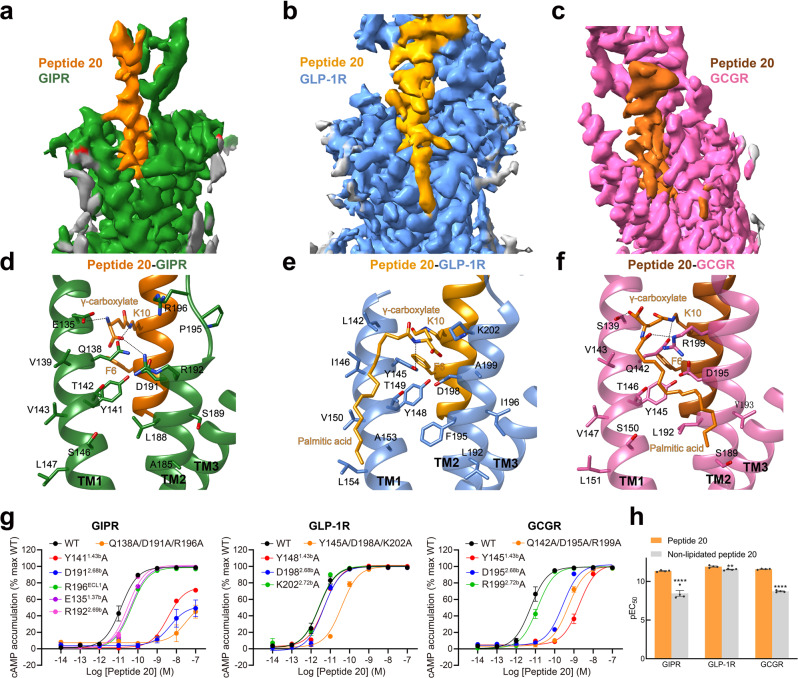
Fig. 5. Structural and functional feature of lipidated K10P of peptide 20.
a–c Close-up views of the crevices between TM1 and TM2 displayed by cryo-EM maps of peptide 20-bound GIPR a, GLP-1R b, and GCGR c. Continuous electron densities connected to K10 in peptide 20 were observed in the three peptide 20-bound receptor–Gs complexes. d–f Interactions between lipidated K10P and the TM1-TM2 crevice of GIPR d, GLP-1R e, and GCGR f, with interacting residues shown in sticks. Hydrogen bonds are shown with dashed lines. g Effects of receptor mutations on peptide 20-induced cAMP accumulation. Data shown are means ± S.E.M. of at least three independent experiments (n = 3–9) performed in quadruplicate. h Effects of K10 lipidation on peptide 20-induced cAMP accumulation. The bar graph represents the average pEC50 (that is, −logEC50) and data are presented as means ± S.E.M. of four independent experiments (n = 4) performed in quadruplicate. Statistically significant differences were determined with a two-tailed Student’s t test. **P < 0.01 and ****P < 0.0001. WT, wild-type. Source data are provided as a Source Data file.