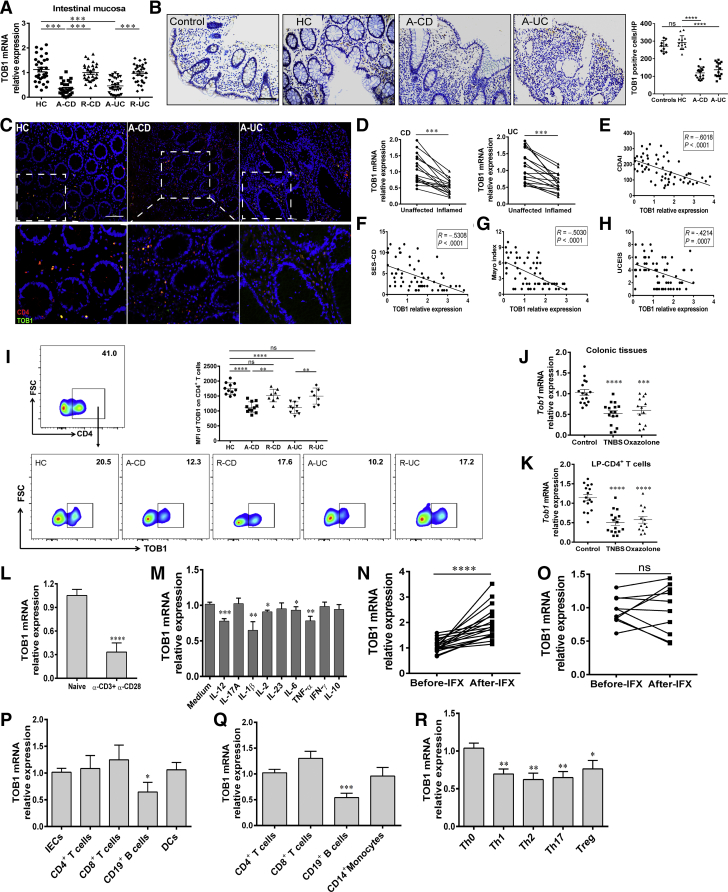
Figure 1.
TOB1 expression is decreased in active IBD patients. (A) Colon biopsies were collected from 29 patients with A-CD, 29 patients with R-CD, 31 patients with A-UC, 30 patients with R-UC, and 31 HC subjects, respectively, and TOB1 expression was analyzed by qRT-PCR. (B) Immunohistochemistry (IHC) analysis of TOB1 in representative sections from colon mucosa of non-IBD inflammatory control subjects (n = 9, including 3 patients with celiac disease, 3 patients with Clostridium difficile infectious colitis, and 3 patients with ischemic enteritis), HC subjects (n = 13), patients with A-CD (n = 17), and patients with A-UC (n = 16), respectively. Scale bars = 100 μm. (C) Immunofluorescence double staining showed co-localization of TOB1 with CD4+ cells in HC, A-CD, and A-UC samples, respectively. Scale bars = 100 μm. (D) TOB1 expression in inflamed and unaffected colon tissues from the same patients with active CD (n = 16) and active UC (n = 17), respectively, was examined by qRT-PCR. (E–H) TOB1 expression in intestinal mucosa of CD patients was observed to be negatively associated with CDAI and Simple Endoscopic Score for Crohn’s Disease (R = –0.6018, P < .0001; and R = –0.5308, P < .0001, respectively) and the correlation analysis between Mayo score, Ulcerative Colitis Endoscopic Index of Severity, and TOB1 expression in intestinal mucosa of UC patients (R = –0.5030, P < .0001; and R = –0.4214, P = .0007, respectively). (I) PB-CD4+ T cells were obtained from HC subjects (n = 11), patients with A-CD (n = 11), patients with R-CD (n = 8), patients with A-UC (n = 10), and patients with R-UC (n = 7), respectively. TOB1 expression was analyzed by flow cytometry, and MFIs (Median Fluorescence Intensity) of TOB1 expression were shown in each group. (J and K) Colon tissues and LP-CD4+ T cells were collected from TNBS- (n = 16), oxazolone-induced (n = 13) colitis mice and WT control animals (n = 16), respectively, and Tob1 expression was examined by qRT-PCR. (L) Naïve PB-CD4+ T cells were sorted from 13 healthy donors and cultured with plate-bound anti-CD3 mAb (5 μg/mL) and soluble anti-CD28 mAb (2 μg/mL) for 24 hours, and qRT-PCR was performed to analyze TOB1 expression. ∗∗P < .01 vs naïve CD4+ T cells. (M) PB-CD4+ T cells (5 × 105/mL) from healthy donors (n = 10) were stimulated with various cytokines (10 ng/mL) for 48 hours, and TOB1 expression was analyzed by qRT-PCR. Gene expression was normalized to GAPDH in each group. (N and O) 29 patients with active CD were receiving anti-TNF mAb (ie, infliximab, IFX) treatment at weeks 0, 2, and 6, and intestinal mucosal biopsies were collected from these patients at week 14 after an induction therapy, (N) 19 patients achieved clinical remission, and (O) 10 patients failed to IFX therapy. (P) Expression of TOB1 mRNA in intestinal mucosal biopsies was analyzed by qRT-PCR. LP-CD4+, CD8+ T, CD19+ B cells, dendritic cells (DCs), and intestinal epithelial cells (IECs) were isolated from normal colon tissues of 6 patients who underwent colectomy for colon cancer, and the mRNA levels of TOB1 were analyzed by qRT-PCR. ∗P < .05 vs IECs. (Q) CD4+, CD8+ T, CD19+ B cells, and CD14+ monocytes were isolated from PB of 8 healthy donors by immunomagnetic positive selection, and TOB1 expression was determined by qRT-PCR. ∗∗∗P < .001 vs CD4+ T cells. (R) PB-CD4+ T cells were isolated from 6 healthy donors and cultured with plate-bound anti-CD3 mAb (5 μg/mL) and soluble anti-CD28 mAb (2 μg/mL) under Th0, Th1, Th2, Th17, and Treg cell–polarizing conditions, respectively, for 5 days, and qRT-PCR was performed to analyze TOB1 expression in these CD4+ T cells. ∗P < .05, and ∗∗P < .01 vs Th0 cells. Spearman rank correlation was used to analyze the correlation between TOB1 expression and disease activities of IBD patients. Statistical analysis was performed with paired 2-sided Student’s t tests or analysis of variance. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001.