Summary
β,γ-Unsaturated α-ketoesters prove to be versatile organic synthons participating in diverse catalytic asymmetric transformations with the breathtaking development of organo-catalysis, new catalytic systems including ingenious chiral ligands as well as Lewis acid cations. The highly efficient creation of stereogenic centers with excellent enantioselectivity is not a surprise, but owes to the bidentate coordination of its unique 1,2-dicarbonyl motif to artful chiral messenger, establishing a rigid system for the precise chiral-identification of the attack. In the past five years, various reaction modes of β,γ-unsaturated α-ketoesters have been developed, involving their multiple reaction sites, such as the carbon-carbon double bond (C=C), the carbonyl group (C=O), the entire C=C-C=O fragment, and the ester group. In this review, we summarize the state-of-the-art catalytic asymmetric reactions of β,γ-unsaturated α-ketoesters, to provide an updated overview to chemists working in this and related fields, facilitating their discoveries in asymmetric catalysis, natural products synthesis, and drug development.
Subject areas: Chemistry, Organic chemistry, Organic synthesis, Organic reaction
Graphical abstract

Chemistry; Organic chemistry; Organic synthesis; Organic reaction
Introduction
Rapid preparation of complex structures from readily accessible starting materials with multiple reaction modes in a catalytic asymmetric manner is one of the central themes in synthetic chemistry. The resulting chiral molecules with structural complexity and diversity are not only important synthons in organic synthesis, but also of great significance and value for hit-to-lead optimization efforts in medicinal chemistry (Kim and Li, 2020; Mei et al., 2021; Xiao et al., 2020). In this context, β,γ-unsaturated α-ketoesters are a class of privileged unsaturated carbonyl derivatives, because of their unique 1,2-dicarbonyl system. This large conjugated system has a specific advantage of multiple reactive sites, which could diversify the reaction modes and broaden their applications in organic synthesis. Besides, the bidentate coordination of 1,2-dicarbonyl motifs with chiral catalysts could increase the reactivity of substrates and the enantio-control of reactions. This intrinsic property makes them popular precursors in asymmetric catalysis for efficient creation of stereogenic centers. Consequently, β,γ-unsaturated α-ketoesters have emerged as attractive and versatile synthons in modern organic synthesis.
Structurally, the presence of the adjacent ester group greatly contributes to the electrophilicity of the ketonic carbonyl group and its neighboring carbon-carbon double bond. The combination of this intrinsic electronic feature and their particular conjugated system results in a rich chemistry of β,γ-unsaturated α-ketoesters involving the carbon-carbon double bond (C=C), the carbonyl group (C=O), the entire C=C-C=O fragment, or the ester group (Figure 1). Typically, β,γ-unsaturated α-ketoesters are employed as good acceptors in 1,4-addition reactions of the C=C bond and 1,2-addition reactions of the C=O group. And the resulting chiral ketoester compounds and tertiary alcohols are versatile chiral building blocks in organic synthesis. In addition, β,γ-unsaturated α-ketoesters are useful and appealing precursors for chiral cyclic compounds. For instance, they can serve as two-atom (2A) synthons in various [2 + n] annulation reactions by making use of the C=C bond or the C=O group. Besides, by reacting with dinucleophiles (Nu-Nu), β,γ-unsaturated α-ketoesters function as dual electrophilic reagents (E-E) in [3 + n] annulation reactions. When it comes to the entire C=C-C=O moiety, β,γ-unsaturated α-ketoesters act as ambident reagents (4A) to afford multiple [4 + n] annulation reactions. Finally, their ester group involved transformations further enrich the reaction modes of β,γ-unsaturated α-ketoesters.
Figure 1.
The reaction modes of β,γ-unsaturated α-ketoesters in asymmetric catalysis
The past decades have witnessed significant advancements in this field. In 2013, Desimoni and coworkers reviewed the earlier examples on catalytic asymmetric reactions of β,γ-unsaturated α-ketoesters (Desimoni et al., 2013). Then, Eftekhari-Sis and coworkers demonstrated their power in the synthesis of heterocycles in 2015 (Eftekhari-Sis and Zirak, 2015). With the breathtaking development of chiral ligands and catalysis, the β,γ-Unsaturated α-ketoesters have become versatile organic synthons over the years, especially in asymmetric synthesis. In this timely review, we would like to summarize the latest developments of catalytic asymmetric reactions of β,γ-unsaturated α-ketoesters from 2015, to provide an updated overview to chemists working in this and related fields, facilitating their discoveries in asymmetric catalysis, natural products synthesis, and drug development. We are paying close attention to the diversified reaction modes of β,γ-unsaturated α-ketoesters. This review is categorized accordingly, including 1,4-addition, 1,2-addition, [2 + n] annulation, [3 + n] annulation, [4 + n] annulation, and others.
Catalytic asymmetric 1,4-addition reactions
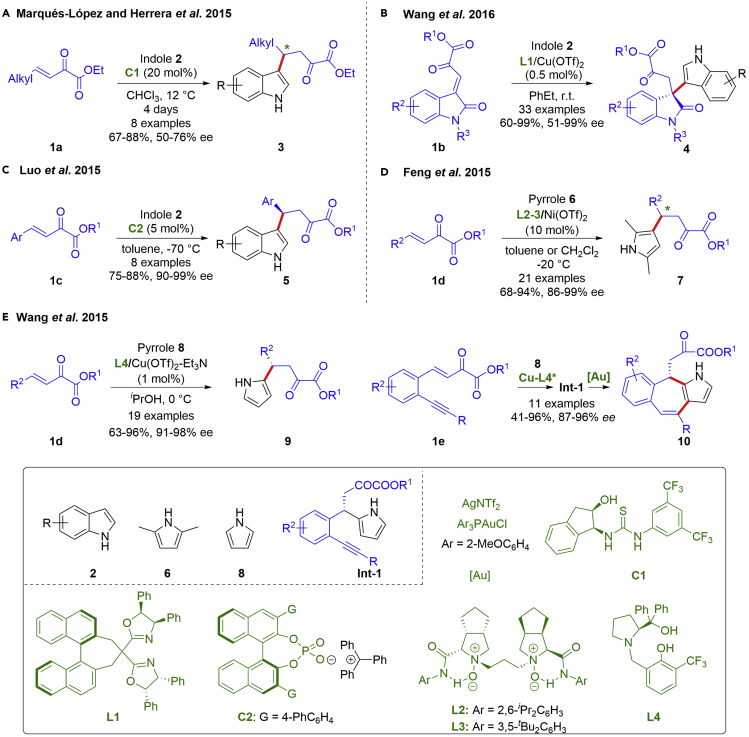
Indole derivatives are fascinating and important heterocyclic compounds, owing to their wide occurrence in a large number of natural products and pharmacologically active agents. The catalytic asymmetric Friedel-Crafts reaction of indoles proves to be one of the most direct approaches to enantiomerically enriched indole compounds. In this context, β,γ-unsaturated α-ketoesters have emerged as particularly interesting activated electrophiles, in terms of their good electrophilicity and easy further manipulation at late stage. In 2015, Marqués-López, Herrera and coworkers developed a thiourea-catalyzed Friedel-Crafts reaction of indoles with aliphatic β,γ-unsaturated α-ketoesters (Scheme 1A) (Juste-Navarro et al., 2015). The authors highlighted the use of aliphatic substrates, which are usually overlooked in comparison with the commonly reported aromatic β,γ-unsaturated α-ketoesters. Under mild reaction conditions, the Friedel-Crafts adducts were obtained in good yields (67–88%) and moderate enantiomeric excess (50–76% ee). In 2016, the Wang group demonstrated that the Friedel-Crafts reaction of indoles with isatin-derived β,γ-unsaturated α-ketoesters 1b could be employed as a practical method for chiral bis-indole products 4 bearing a quaternary stereocenter, a kind of naturally occurring motifs (Scheme 1B) (Li et al., 2016b). Chiral BINOL-derived bisoxazoline (BOX)/copper (II) complex was evaluated as the best catalyst with a low loading of 0.5 mol%. The desired products were obtained in excellent yields (60–99%) and enantioselectivities (51–99% ee), which could be transformed to α-amino esters, α-hydroxy esters and α-keto amides.
Scheme 1.
Catalytic asymmetric Friedel-Crafts reaction with electron-rich heterocycles
In light of the significance of asymmetric Friedel-Crafts reaction of indoles with β,γ-unsaturated α-ketoesters in organic synthesis, diversified catalytic systems were developed to guarantee the reaction efficiency and enantioselectivity. For instance, Luo et al. found that the asymmetric carbocation catalysis with chiral trityl phosphate could be utilized as an effective strategy for the Friedel-Crafts reaction between unprotected indoles 2 and β,γ-unsaturated α-ketoesters 1c (Scheme 1C) (Lv et al., 2015). It was reported that the catalytically active chiral ion pair for substrate activation and chiral induction was generated in situ via mixing chiral trityl phosphate with Ph3CBr. The standard conditions were applicable to various aromatic β,γ-unsaturated α-ketoesters 1c, giving the desired 1,4-addition products 5 in good yields (75–88%) with high enantioselectivities (90–99% ee). Besides, heterogeneous catalysts such as metal-organic frameworks (MOF) (Chen et al., 2017) and polystyrene-supported BOX-Cu(II) complex (Desyatkin et al., 2017), also showed high-performance in this kind of asymmetric Friedel-Crafts reactions.
On the other hand, pyrroles are another class of important aromatic feed stocks; however, they are relatively less explored in catalytic asymmetric Friedel-Crafts reaction, likely due to the regioselectivity and reactivity issues. In 2015, Feng et al. disclosed an asymmetric Friedel-Crafts C3-alkylation of 2,5-dimethyl pyrrole 6 to β,γ-unsaturated α-ketoesters 1d, wherein the chiral Ni(II)-complexes of N,N′-dioxides exhibited high catalytic performance (Scheme 1D) (Zhang et al., 2015). Notably, a dramatic reversal of enantioselectivity was achieved by slightly tuning the amide units of the same type of chiral N,N′-dioxides. By using this approach, the two sets of enantiomers 7 were obtained in high yields (68–94%) and enantioselectivities (86–99% ee). Moreover, the substrate scope could be extended to indoles. In the same year, a highly enantioselective, efficient Friedel-Crafts C2-alkylation of pyrrole 8 with β,γ-unsaturated α-ketoesters 1d was accomplished by the Wang group (Scheme 1E) (Hu et al., 2015). Under the catalysis of the chiral Cu-prolinol derivative complex, good to excellent yields (63–96%) and excellent enantioselectivities (91–98% ee) were achieved. Furthermore, a one-pot construction of the seven-membered ring fused with pyrroles 10 from delicately designed β,γ-unsaturated α-ketoesters 1e was developed by virtue of the dual metal system of copper and gold catalysis.
In addition to Friedel-Crafts reactions with aromatic compounds, non-aromatic nucleophiles have been well employed as Michael donors for 1,4-addition reactions of β,γ-unsaturated α-ketoesters. For instance, nitro-compounds are useful and appealing nucleophilic reagents, because the nitro group could readily stabilize carbanions via the conjugate effect and possess good potential for further functional group transformations. In 2015, the Yan group further developed the organo-catalyzed asymmetric 1,4-addition reaction between β,γ-unsaturated α-ketoesters 1f and nitroacetate 11 (Scheme 2A) (Liu et al., 2015). The following decarboxylation of the primary adducts was achieved via refluxing over silica gel. The nitro-products 12 were yielded in excellent enantioselectivities (86–96% ee) and could be transformed into optically active proline derivatives. Recently, Bonne and Bugaut et al. disclosed that cyclic β,γ-unsaturated α-ketoesters 1g could serve as suitable acceptors for the asymmetric 1,4-addition reaction with nitroalkanes 13 (Scheme 2B) (Fofana et al., 2020).
Scheme 2.
Organo-catalyzed asymmetric Michael-addition
Furthermore, chiral indoline derivatives are important structural motifs and building blocks. Extensive attention from both synthetic and medicinal chemists has been attracted. Among them, the asymmetric Michael addition of indole-containing substrates to β,γ-unsaturated α-ketoesters has been regarded as one straightforward and powerful protocol. In 2016, the Zhou group reported an asymmetric Michael addition of 1-acetylindolin-3-ones 15 to β,γ-unsaturated α-ketoesters 1d, delivering chiral indolin-3-ones 16 with two adjacent tertiary stereogenic centers in high yields (56–99%) with excellent diastereo- and enantioselectivities (91–99% ee, >19:1 dr) mild reaction conditions (Scheme 2C) (Chen et al., 2016). Asymmetric vinylogous Michael addition poses a daunting challenge in organic synthesis, because of the distal stereocontrol. Recently, Singh et al. disclosed that a broad range of enantio-enriched oxindoles 18 could be well synthesized via a highly selective asymmetric vinylogous Michael addition of 3-alkylidene-2-oxindoles 17 to β,γ-unsaturated α-ketoesters 1f (Scheme 2D) (Jaiswal et al., 2020). In addition, α-azido indanones 19 are valuable structural scaffolds, in view of their easy and useful handle for synthesizing nitrogen-containing molecules and heterocycles. In 2020, the diastereodivergent catalytic enantioselective Michael addition of 19 to 1d was described by Yu and Zhou et al. (Scheme 2E) (Ding et al., 2020). Interestingly, additive hexafluoroisopropanol (HFIP) played a crucial role to achieve the diastereoselectivity reversal. Although the merger of C7 and HFIP afforded syn-adducts 20, anti-adducts 20 were dominant without the aid of HFIP. Valuable α-chiral tertiary azides were afforded with high efficiency and readily converted to enantioenriched spiro N-heterocycles.
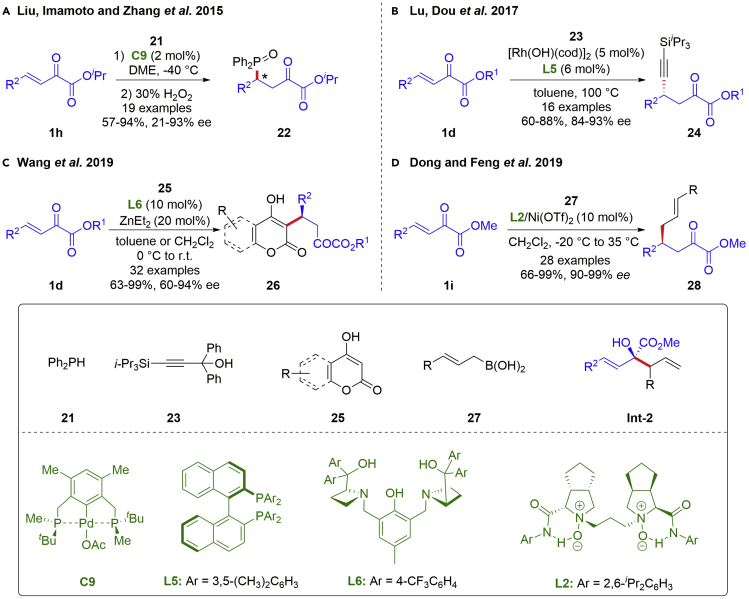
Despite the many progresses made in organo-catalyzed versions, metal-catalyzed conjugate 1,4-additions of β,γ-unsaturated α-ketoesters with various nucleophiles satisfy more diversified demand for α-keto carbonyl compounds bearing a γ-chiral center. In 2015, the Zhang group reported an asymmetric Michael addition of diphenylphosphine 21 to β,γ-unsaturated α-ketoesters 1h, using their developed P-stereogenic pincer-Pd complex as the catalyst (Scheme 3A) (Xu et al., 2015). The corresponding products 22, phosphonate-containing compounds, were generated in good yields (57–94%) with moderate to good enantioselectivities (21–93% ee). Asymmetric conjugate addition of sp-carbon nucleophile to β,γ-unsaturated α-ketoesters remains elusive and challenging. In 2017, the first example of catalytic asymmetric conjugate alkynylation of β,γ-unsaturated α-ketoesters was accomplished by the Dou group (Scheme 3B) (Zhi et al., 2017). By using a chiral rhodium catalytic system, diphenyl [(triisopropylsilyl)ethynyl]methanol 23 acted as the alkynylating reagent generated in situ. The alkynylation products 24 bearing a propargylic chiral center at γ-position were created in good yields (60–88%) with high enantioselectivities (84–93% ee). Latter, the heterogeneous chiral rhodium nanoparticle system with a chiral diene ligand was proven to be effective for the asymmetric conjugate 1,4-addition of arylboronic acids (Miyamura et al., 2019). Featured by Lewis acidic and Brønsted basic sites, dinuclear Zinc-Pro-Phenol complex has emerged as a powerful catalyst to simultaneously activate both nucleophile and electrophile in the same chiral environment, facilitating a broad range of asymmetric C-C bond formations (Trost et al., 2020). In 2019, azetidine-derived dinuclear Zinc-catalyzed asymmetric conjugate addition of 4-hydroxyl pyrones or 4-hydroxycoumarins 25 to β,γ-unsaturated α-ketoesters was developed by the Wang group (Scheme 3C) (Liu et al., 2019). The protocol was applicable to a wide range of substrates under mild conditions, leading to biologically important coumarins and pyranones in good to excellent ee values (60–94% ee).
Scheme 3.
Metal-catalyzed asymmetric conjugate additions with other nucleophilic reagents
In addition, metal-catalyzed conjugate additions of β,γ-unsaturated α-ketoesters can be achieved via an unusual process. In spite of the well-developed racemic 1,4-allylations, such catalytic asymmetric versions are less investigated. In 2019, the Feng group reported a highly enantioselective formal conjugate allyl addition of allylboronic acids 27 to β,γ-unsaturated α-ketoesters 1i, providing a facile route to γ-allyl-α-ketoesters with good yields (66–99%) and excellent ee values (90–99%) (Scheme 3D) (Tang et al., 2019). In the presence of chiral NiII/N,N′-dioxide complex, this transformation readily occurred via an allylboration/oxy-Cope rearrangement sequence, which was confirmed by the isolation of 1,2-allylboration products Int-2. Recently, a series of enantioenriched γ-allyl (aryl)-α-ketoesters was well synthetized by Chai and Chang et al. via (R)-3,3′-Br2-BINOL-catalyzed enantioselective Michael addition of organic boronic acids to β,γ-unsaturated α-ketoesters (not shown) (Zhao et al., 2021).
Catalytic asymmetric 1,2-addition reactions
In conjugated systems, 1,2-addition reactions are challenging and commonly regarded as competing reactions to 1,4-addition reactions. However, these concerns could be addressed by selection of suitable catalytic systems and matching reactants. Ketones proved to be a class of versatile nucleophiles for construction of C-C and C–X (X = N, O, S, Cl, Br etc.) bonds, thanks to the facile deprotonation at the α-position. Among these transformations, the asymmetric catalytic aldol condensation gathered much attention, revealing a dependable access to chiral β-hydroxy carbonyl compounds. Recently, great progress has been made in asymmetric aldol reactions of ketones with β,γ-unsaturated α-ketoesters. In 2015, Zhao and coworkers developed an enantioselective aldol addition of acetophenones 29 to β,γ-unsaturated α-ketoesters 1c (Scheme 4A) (Konda et al., 2015). The desired aldol products 30 were obtained in high yields (70–99%) with moderate to excellent ee values (50–91% ee), under the catalysis of a quinidine-derived thiourea. Notably, the substrate concentration showed a marked impact on the reactivity. Only a small amount of THF was necessary, because lower yield and ee value was received in dilute or neat reaction condition. In addition, this presented protocol could be extended to the aldol reaction of phenylglyoxal hydrates and cyclic ketones (not shown).
Scheme 4.
Catalytic asymmetric 1,2-addition reactions
In the same year, a chiral Ni (II) complex C10 catalyzed enantioselective decarboxylative aldol reaction of β,γ-unsaturated α-ketoesters 1d with β-ketoacids 31 was described by Ma et al. to synthesize highly functionalized chiral tertiary alcohols 32 (Scheme 4B) (Wei et al., 2015). The transformation proceeded smoothly for a broad range of β-ketoacids and β,γ-unsaturated α-ketoesters with high chemo-, regio-, and enantioselectivities (65–99% ee). The 13C NMR spectroscopic indicated one of the diamine ligands could be released from the Ni-complex C10 to give way to β-ketoacids 31 instead of β,γ-unsaturated α-ketoesters 1d, and the liberated diamine could then deprotonate the metalbound β-ketoacid. In 2006, enzyme catalysis was applied to the asymmetry aldol reaction between β,γ-unsaturated α-ketoesters and aliphatic ketones by He and Guan et al. (Li et al., 2016c). Although enantioselectivities were not ideal, as an environment-friendly and sustainable biocatalyst, the enzyme catalysis showcased a wide application future in this field.
Addition to the versatile reactivity, coumaran-3-ones exhibit the importance in synthesis, for their unique motif widely displayed in diverse natural products and intermediates with pharmaceutical and biological activities. Lately, an efficient copper-catalyzed asymmetric aldol reaction between β,γ-unsaturated α-ketoesters 1c and coumaran-3-ones 33 was developed by Zhang and Wang et al. (Scheme 4C) (Li et al., 2021). The desired chiral tertiary alcohol derivatives 34 containing the coumaran-3-one structure were obtained in excellent yields (87–96%) and stereoselectivities (84–95% ee) with a wide range of functional group tolerance, offering a great latitude for further structural elaborations for biologically important chiral coumaran-3-one derivatives. In the same year, Hu and Jiang et al. described a decarboxylative aldol reaction of β,γ-unsaturated α-ketoesters 1c with 2-oxotetrahydrofuran-3-carboxylic acid 35/2-oxochromane-3-carboxylic acid derivatives 37 (Scheme 4D) (He et al., 2021b). The desired product α-substituted β-hydroxy butyrolactones 36 and dihydrocoumarins 38 were well generated in the presence of a bidentate oxazoline-Ni(II) catalysis. High levels of functional-group compatibility, reaction yields (59–83%), diastereo- and enantioselectivities (74–99% ee, 2:1 to 12:1 dr) were received. The high resolution mass spectroscopy (HRMS) analysis of the reaction indicated that the bidentate coordination existed between the Ni(II) and 2-oxotetrahydrofuran-3-carboxylic acid 35 and decarboxylation might occur after the aldol addition.
β-Nitroalcohol derivatives are intermediates of value, which could take part in diverse transformations to produce useful nitrogen-containing compounds conveniently, such as aminoalcohols, unnatural sugars, and nitroolefins. In this regard, the asymmetric Henry reaction has attracted much attention and been widely applied in the construction of β-nitroalcohol motifs. In 2017, the Wang groups reported a chiral copper complex catalyzed highly enantioselective Henry reaction of β,γ-unsaturated α-ketoesters 1d with nitromethane in water (Scheme 4E) (Li et al., 2017b). A series of unsaturated β-nitro-α-hydroxy esters 40 bearing tetrasubstituted carbon stereocenters were gathered in high yields (75–91%) with excellent ee values (83–96% ee). In 2019, Wu and Han et al. described a enantioselective Henry reaction of β,γ-alkynyl ketoesters 1j with nitromethane catalyzed by tartaric acid derived chiral iminophosphoranes C11 (Scheme 4F) (Zhang et al., 2019b). The desired products β-nitroalcohols 41 were generated in moderate to excellent yields (77–99%).
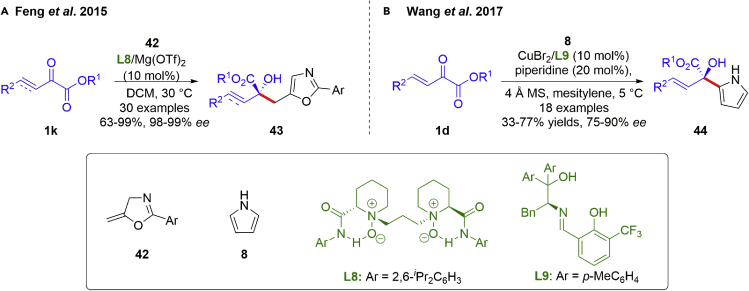
Besides, chiral alcohols with heteroaromatics are a class of important pharmaceutical intermediates, and widely distributed in natural products, microbial and bioactive molecules. Metal-catalyzed asymmetric 1,2-additions of β,γ-unsaturated α-ketoesters with heterocycles represent as a practicable synthetic method. The synthesis of chiral tertiary alcohols bearing oxazole fragments 43 was developed by the Feng group in 2015 via a catalytic asymmetric carbonyl-ene reaction of β,γ-unsaturated α-ketoesters 1k with 5-methyleneoxazolines 42 (Scheme 5A) (Luo et al., 2015). The transformation was facilitated by the utilization of a chiral N,N′-dioxide/MgII catalyst, giving the desired products in good to excellent yields (63–99%) with excellent enantioselectivities (98–99% ee) under mild reaction conditions. The afforded results demonstrated a high level of functional-group compatibility for both β,γ-alkenyl α-ketoesters and β,γ-alkynyl α-ketoesters. It was proposed that β,γ-unsaturated α-ketoesters could be coordinated to the MgII in a bidentate fashion with its dicarbonyl motif, and the steric hindrance on the phenyl ring of the ligand played a key role in promoting both the enantioselectivity and reactivity. Pyrroles could readily react with various electrophilic reagents bearing prochiral centers to give enantiomerically enriched pyrrole derivatives, taking advantage of the electron-rich aromatic rings. In 2017, the Wang group reported a copper catalyzed asymmetry 1,2-addition reaction of β,γ-unsaturated α-ketoesters 1d with pyrrole, as opposite to the previous 1,4-addition (Scheme 5B) (Sun et al., 2017). The desired products 44 were obtained in moderate to good yields (33–77%) with excellent enantioselectivities (75–90% ee), under the catalysis of copper-complex.
Scheme 5.
Metal-catalyzed Asymmetric 1,2-addition reactions involving heterocycles
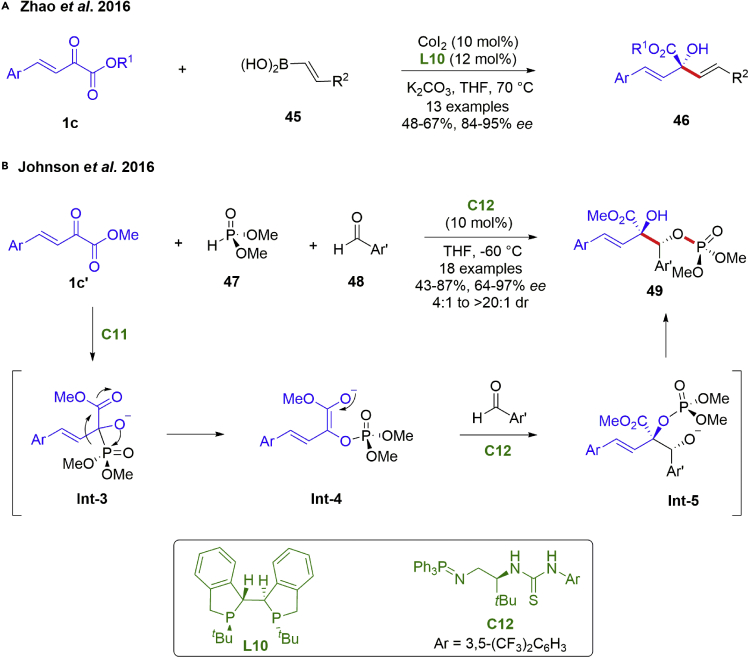
In spite of above-mentioned achievements, 1,2-addition reactions with allylic nucleophiles remain underdeveloped. The products allylic alcohols are among the most abundant and significant structural motifs in organic synthesis, which could be facilely converted to a range of stereodefined compounds of value in chemistry and medicine in a stereoselective fashion. In 2016, The Zhao group developed an unprecedented vinylation of β,γ-unsaturated α-ketoesters 1c using vinyl boronic acid reagents 45 (Scheme 6A) (Huang et al., 2016b). The reactions were carried out in the presence of commercially available cobalt halides and chiral bisphosphine ligands. Transmetalation of vinyl boronic acid with cobalt halide was facilitated by K2CO3, and followed by an enantioselective nucleophilic addition to the carbonyl group of β,γ-unsaturated α-ketoesters, affording the desired highly optically enriched (84–95% ee) allylic alcohols 46. It is noteworthy that this catalytic system could be further expanded to the asymmetry vinylation of α-ketoesters, isatins, and imines. Recently, the Li group revealed that Bi(OAc)3/chiral phosphoric acid catalyst system also showed high catalytic and stereocontrolling abilities in this kind of asymmetric 1,2-allylation reaction of allylboronic acid pinacol esters to β,γ-unsaturated α-ketoesters (Liu et al., 2021). In addition, the Johnson group completed a three-component reductive coupling reaction between β,γ-unsaturated α-ketoesters, dimethyl phosphite and aldehydes (scheme 6B) (Horwitz et al., 2016). The transformation was conducted by a chiral triaryliminophosphorane C12, offering the desired products hydroxy phosphates 49 in high yields (43–87%) with excellent diastereoselectivities and enantioselectivities (64–97% ee, 4:1 to 20:1 dr). It was proposed that the transformation proceeded a Pudovik addition of a dimethyl phosphite to β,γ-unsaturated α-ketoesters, triggering the phospha-Brook rearrangement and subsequent catalyst controlled trapping of the resultant enolate Int-4 with an aldehyde 48.
Scheme 6.
Catalytic asymmetric 1,2-additions with other nucleophilic reagents
Catalytic asymmetric [2 + n] annulation reactions
Although 1,4-and 1,2-additions with diversified nucleophiles lead to straightforward and powerful protocols for enantio-enriched ketoesters and tertiary alcohols, multiform cyclization reactions including [2 + n], [3 + n] and [4 + n] annulation afford efficient and convenient approaches to various ring skeletons, as such structural motifs are widely present in medicinal chemistry and natural products.
Catalytic asymmetric [2 + 1] annulation reactions
Enantio-enriched cyclopropanes are common structural moieties in natural products and pharmacologically active agents. Despite the tremendous achievement that has been made, the synthesis of these molecules still meets challenges, possibly because of the ring strain. In this context, β,γ-unsaturated α-ketoesters proved to be versatile precursors for chiral cyclopropanes. In 2017, the Luo group completed an enantioselective cyclopropanation of β,γ-unsaturated α-ketoesters with diazoesters catalyzed by the complex of InBr3 and chiral calcium phosphate (scheme 7A) (Zhong et al., 2017). β,γ-Unsaturated α-ketoesters were activated by bidentate coordination with the cationic indium complex, setting a rigid system for the chiral-identification. Weak π-interaction between chiral phosphoric acid and diazo ylide was supposed to contribute to a facial attack and the following back attack cyclization with loss of nitrogen. Highly functionalized chiral cyclopropanes 51 were prepared in moderate to good yields (54–77%) with excellent ee values (99% ee) as a single diastereoisomer. A demonstrable fluoro substitution effect was observed in this binary Lewis acid catalysis while increasing the number of fluoro substituent on the ligand dramatically benefited the enantioselective control. The reactions were regarded to proceed via a 1,4-addition-cyclopropanation sequence. Notably, Chen and Xiao highlighted the cooperative effect of the hydrogen-bond on stereoinduction origination in the chiral ureas catalyzed asymmetric cyclopropanation of β,γ-unsaturated α-ketoesters with stabilized sulfur ylides in 2011 (not shown) (Cheng et al., 2011). In 2018, Liu and Feng et al. described a diastereodivergent asymmetric Michael-alkylation between 3-Cl oxindoles and β,γ-unsaturated-α-ketoesters (scheme 7B) (Kuang et al., 2018). By tuning metal catalysts and adjusting the ligands and temperature, rel-(1R,2S,3R) and rel-(1S,2S,3R) spiro cyclopropane oxindoles were obtained separately in high yields (50–99%) with excellent enantioselectivities (72–99% ee). It is proposed that the rel-(1S,2S,3R) diastereoisomers 53 were obtained via a intramolecular trapping of the chiral aza-ortho-xylylene intermediate Int-6 after Michael addition, whereas the other diastereoisomers (iso)-53 were generated from Int-7 via a direct SN2 substitution pathway, which was because of the different characteristics of the metal catalysts.
Scheme 7.
Catalytic asymmetric [2 + 1] annulation reactions of β,γ-unsaturated α-ketoesters
Catalytic asymmetric [2 + 3] annulation reactions
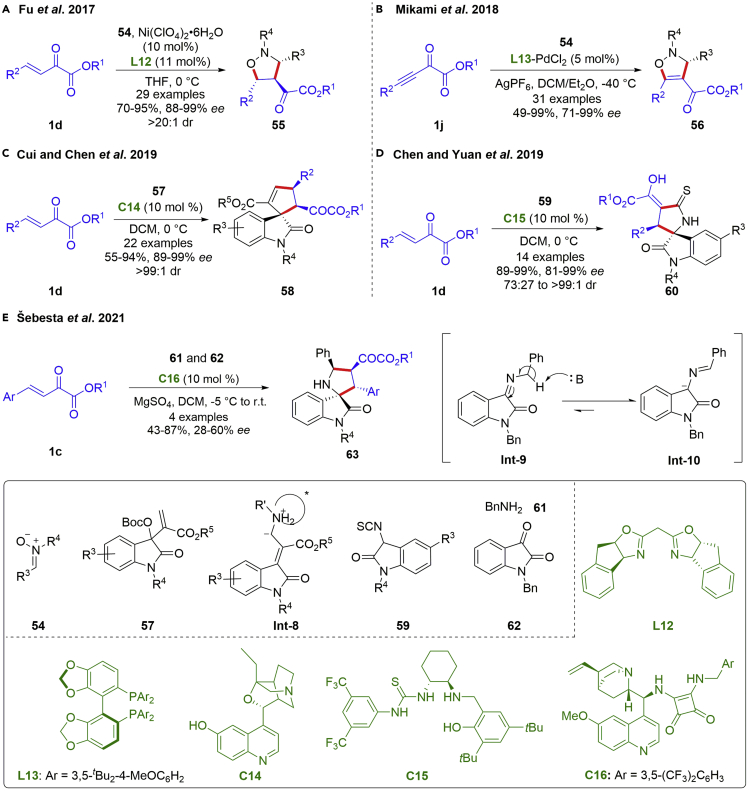
Over the past decades, the asymmetry 1,3-dipolar cycloaddition of nitrones has become one of the most powerful synthetic methods for enantio-enriched five-membered heterocycles. In this regard, β,γ-unsaturated α-ketoesters involving the electron-deficient carbon-carbon double bond proved to be useful acceptors for 1,3-dipolar cycloaddition. A highly stereoselective 1,3-dipolar cycloaddition of β,γ-unsaturated α-ketoesters with nitrones was presented by the Fu group in 2017 (scheme 8A) (Xie et al., 2017). The reaction was conducted by a chiral bis(indano-oxazoline)-based Ni complex to provide isoxazolidines 55 possessing three contiguous stereocenters in high yields (70–95%) with excellent diastereoselectivities and enantioselectivities (88–99% ee, >20:1 dr). This method showcased a reliable approach toward the synthesis of isoxazolidines, γ-amino alcohols and other related bioactive derivatives. Later, enantioselective [2 + 3] cycloaddition of β,γ-alkynyl ketoesters and nitrones has been developed by the Mikami group, using a cationic chiral Pd(II) Lewis acid catalyst (Scheme 8B) (Honda and Mikami, 2018).
Scheme 8.
Catalytic asymmetric [2 + 3] annulation reaction involved β,γ-C=C bond
Meanwhile, the development of new synthetic methods of 3,3′-disubstituted oxindoles is of considerable interest in view of their widespread occurrence in natural products, drugs, and pharmaceutically active compounds. In 2019, Cui and Chen et al. accomplished a chiral tertiary amine catalyzed enantioselective [2 + 3] annulation reaction of β,γ-unsaturated α-ketoesters and 1,3-dipole Int-8 generated in situ from isatin-derived MBH carbonates 57 via addition-eliminations (scheme 8C) (Chen et al., 2019). The transformation proceeded a nucleophilic α-attack of the Int-8 to β,γ-unsaturated α-ketoesters via the hydrogen bonding activation, followed by an intramolecular conjugate addition cyclization. An allylic deprotonation was facilitated by base to give the catalysis regeneration. A various of spirocyclic compounds 58 with three contiguous stereocenters were obtained in moderate to high yields (55–94%) with excellent diastereo- and enantioselectivities (89–99% ee, >99:1 dr). In the same year, Chen and Yuan et al. completed an asymmetric domino Mannich-cyclization of β,γ-unsaturated α-ketoesters 1d with 3-isothiocyanato oxindoles 59 (scheme 8D) (Bai et al., 2019). The desired products spirocyclic oxindoles 60 incorporating 3,3′-disubstituted oxindole frameworks were gathered in high yields (89–99%) with good to excellent diastereo- and enantioselectivities (81–99% ee, 73:27 to >99:1 dr) under the catalysis of a thiourea-secondary amine. This transformation revealed a valuable method for the synthesis of a wide range of densely functionalized spirocyclooxindole derivatives bearing two contiguous stereogenic centers and enol motifs. Recently, the enantioselective [2 + 3] annulation reactions of β,γ-unsaturated α-ketoesters with keto amides generated in situ from N-benzylisatine 62 and benzylamine had been developed by the Šebesta groups (scheme 8E) (Peňaška et al., 2021). The transformations proceeded smoothly under mild conditions with a chiral quinine-based squaramide catalyst to afford spirocyclic oxindole pyrrolidines 63 in moderate to high yields and ee values (43–87% yields, 28–60% ee). It is noteworthy that the reaction was carried out to mix the N-benzylisatine 62 with benzylamine firstly to prevent the undesired competitive reaction between benzylamine and β,γ-unsaturated α-ketoesters.
Beside the β,γ-C=C bond, a novel [2 + 3] annulation reaction involving α,β-position also attracts much attention. In 2015, the Singh group completed an enantioselective interrupted Feist-Bénary reaction of β,γ-alkynyl α-ketoesters (scheme 9A) (Sinha et al., 2015). Under the catalysis of the chiral phosphine-silver(I) complex, the transformation started with 1,2-addition of 1,3-diketones to β,γ-alkynyl ketoesters, which was followed by a tandem 5-exo-dig cyclization. Dihydrofuran derivatives 65 were obtained in moderate to excellent yields (40–95%) with high values of enantioselectivity (71–98% ee). In addition, in 2019, the Kesavan group described an asymmetric [3 + 2] formal cycloaddition reaction between oxindole α-keto esters 1b and enals 66 (scheme 9B) (Muthusamy and Kesavan, 2019). The reaction progress possibly involved the generation of 1,3-dien-1-amine intermediate Int-13 from enals 66, followed by 1,4-addition to the oxindole α-keto esters 1b, intramolecular cyclization and the final isomerization. By treatment with DAMP and Boc2O in one pot, the final products multi-functionalized carbocyclic oxindoles 67 were separated in moderate yields (62–80%) with good enantioselectivities (65–91% ee).
Scheme 9.
Catalytic asymmetric [2 + 3] annulation reaction involved α,β-position
Catalytic asymmetric [2 + 4] annulation reactions
There is no doubt that the [2 + 4] annulation including carbo- and hetero Diels-Alder reaction is one of most available methods in organic synthesis. Both the electron-deficient carbon-carbon double bond and activated carbonyl group of β,γ-unsaturated α-ketoesters serve as useful acceptors for [2 + 4] annulation reaction. In 2016, Lin and Feng et al. descried a highly efficient catalytic asymmetric [2 + 4] cycloaddition of β,γ-unsaturated α-ketoesters with silyloxyvinylindoles (Scheme 10A) (Zhao et al., 2016). The transformation proceeded smoothly under mild conditions, affording the desired products carbazole derivatives 69 bearing three contiguous stereocenters in high yields (47–98%) with excellent diastereo- and enantioselectivities (86–99% ee, >19:1 dr). It is noteworthy that the steric hindrance of amide substituents on the ligand L15 was crucial for enantiocontrol. Subsequently, a catalytic asymmetric Diels-Alder reaction of β,γ-unsaturated α-ketoesters with (E)-1phenyl dienes 70 was accomplished by the same group in 2018, using a chiral N,N′-dioxide/zinc(II) complex as catalyst (Scheme 10B) (Xiong et al., 2018). Excellent diastereo- and enantioselectivities (88–99% ee, >19:1 dr) were observed. HRMS analysis and operando IR experiments implied that the ZnII center was coordinated to four oxygens of the N,N′-dioxide and two oxygens of β,γ-unsaturated α-ketoester (Int-16) to decrease the LUMO energy of β,γ-unsaturated α-ketoester for the Diels-Alder reaction. The steric hindrance of amide substituents on the ligand occupied a key role to compel the (E)-1-phenyl dienes to attack from the Re-face of the β,γ-unsaturated α-ketoester.
Scheme 10.
Catalytic asymmetric [2 + 4] annulation reactions of C=C bond
On the other hand, great progress also has been made in organo-catalyzed asymmetric [2 + 4] annulation reactions of β,γ-unsaturated α-ketoesters. The catalytic asymmetric aza-Michael-Michael cascade derived from β,γ-unsaturated α-ketoesters and 2-aminochalcones 72 was developed by the Li group to prepare enantiomerically enriched tetrahydroquinoline derivatives 73 in 2018 (Scheme 10C) (Duan et al., 2018). Three contiguous stereogenic centers were created with high enantio- and diastereoselectivities in the presence of a bifunctional squaramide catalyst. The combination of the trityl cation and chiral weakly coordinating metal-based bisphosphate anions was utilized in numbers of novel transformation as a highly active carbocation Lewis acid catalyst by the Luo group in recent years. In 2019, Lv and Luo et al. demonstrated that asymmetric carbocation catalysis with chiral weakly coordinating Fe(III)-based bisphosphate anion was an effective catalytic system for the enantioselective Diels-Alder reactions of β,γ-unsaturated α-ketoesters with unsubstituted anthracene (Scheme 10D) (Zhang et al., 2019a). It was regarded that the catalytically active chiral ion pair for substrate activation and chiral induction was generated in situ via mixing the Fe(III)-based bisphosphate anion with Ph3CCl. The desired annulation products 75 were yielded with moderate to excellent ee values (55–93% ee). In 2020, Liu and Zhou et al. conducted a domino Michael/annulation reaction between β,γ-unsaturated α-ketoesters and bifunctional oxindole-chromones 76 (Scheme 10E) (Zhou et al., 2020). The process was catalyzed by a chiral thiourea under a mild condition to deliver structurally diverse polysubstituted hexahydroxanthones 77 with five contiguous stereogenic centers including one spiro quaternary center in good yields (56–76%) with excellent ee values (93–99% ee).
Catalytic asymmetric [2 + 4] annulation reactions of C=O bond
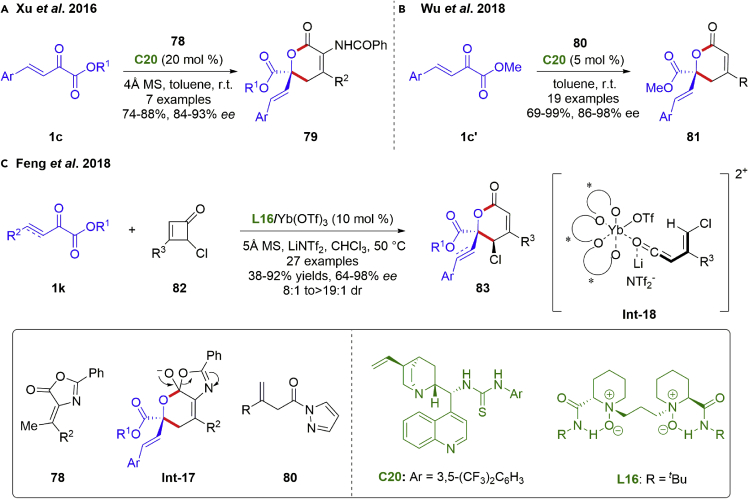
In addition, utilizing the carbonyl group of β,γ-unsaturated α-ketoesters as C2 synthon for asymmetry [2 + 4] annulation reactions proved to be a reliable access to chiral 5,6-dihydropyran-2-ones, which were presented in a series of biologically active compounds. In 2016, the Xu group accomplished an enantioselective organo-catalytic [2 + 4] annulation of β,γ-unsaturated α-ketoesters with dienes generated in situ from olefinic azlactones 78 (Scheme 11A) (Gao et al., 2016). Under the catalysis of tertiary amine-thiourea C20, hetero-Diels–Alder reaction occurred to form Int-17 and followed by an isomerization, affording chiral dihydropyranones 79 in good yields (74–88%) with excellent ee values (84–93% ee). It is noteworthy that this catalytic system was also adapted to the reaction of arylglyoxalates with olefinic azlactones. Similarly, the Wu group achieved the [4 + 2] annulation of β,γ-unsaturated α-ketoesters with β,γ-unsaturated amide (Scheme 11B) (Zhang et al., 2018). In 2018, the Feng group reported an N,N′-dioxide/Yb(III) complex catalyzed asymmetric [2 + 4] cycloaddition of β,γ-unsaturated α-ketoesters with cyclobutenones (Scheme 11C) (Yao et al., 2018). A broad range of 4,5,6-trisubstituted ropyran-2-ones 83 bearing contiguous stereogenic centers were obtained in good yields (38–92%) with excellent enantioselectivities and diastereoselectivities (64–98% ee, 8:1 to >19:1 dr). The Yb3+ was coordinated with the oxygens of amine-oxide and amide of L16 in a tetradentate manner, establishing a chiral pocket. The proposed reaction mechanism involved the generation of the activated cis-vinylketene intermediate Int-18, coordination of 1,2-dicarbonyl motif with Yb(III) complex and the final [2 + 4] annulation occurring between the Re-face of β,γ-unsaturated α-ketoesters and the Si-face of the vinylketene.
Scheme 11.
Catalytic asymmetric [2 + 4] annulation reactions of C=O bond
Catalytic asymmetric [3 + n] annulation reactions
Catalytic asymmetric [3 + 2] annulation reactions
By treatment with dinucleophiles (Nu−Nu), β,γ-unsaturated α-ketoesters could be employed as C3 synthons in [3 + n] annulation reactions, on account of the 1,3-dual electrophilic sites. In 2015, Wang and Chang et al. described a dinuclear zinc-AzePhenol complex catalyzed enantioselective domino Michael/hemiketalization reaction of β,γ-unsaturated α-ketoesters with α-hydroxyacetophenone, showcasing an available access to 2,2,4,5-tetrasubstituted chiral tetrahydrofurans 85 in high yields (59–90%) with good to excellent enantioselectivities (75–98% ee) (Scheme 12A) (Hua et al., 2015). Simple treatment with sulfuric acid converted 85 to corresponding dihydrofurans. In the same year, a catalytic enantioselective Friedele−Crafts alkylation/N-hemiacetalization cascade reaction of β,γ-unsaturated α-ketoesters and 3-methylindoles 86 was described by the Fu group for the preparation of chiral pyrroloindole derivatives 87 (Scheme 12B) (Ma et al., 2015). The reaction was performed with addition of a heteroarylidene-tethered bisoxazoline ligands-copper (II) complex as catalysis to yield the nitrogen-heterocycles with high to excellent enantioselectivities and diastereoselectivities (87–98% ee, 79:21 to >96:4 dr). It is important to note that the reaction of [3 + 2] annulation reactions of substituted indoles and β,γ-unsaturated α-ketoesters was also previously described by Chen and Xiao et al., in 2013 (not shown) (Cheng et al., 2013; Zhu et al., 2012). In 2017, the Wang group disclosed a similar synthetic strategy with catalysis of Pd-complex to construct optically active spiro-polycyclic indoles (Li et al., 2017a). In 2018, the Du group reported an enantioselective Michael/N-hemiketalization cascade reaction of isatin-derived β,γ-unsaturated α-ketoesters and 3-aminooxindoles 88 (Scheme 12C) (Lin et al., 2018). This cinchona-derived bifunctional squaramide catalyzed event afforded various chiral 3,3′-pyrrolidinyl-bispirooxindoles 89 in moderate to good yields (63–85%) with high diastereo- and enantioselectivities (63–91% ee, 2:1 to >19:1 dr) under mild conditions.
Scheme 12.
Catalytic asymmetric [3 + 2] annulation reactions of β,γ-unsaturated α-ketoesters
Catalytic asymmetric [3 + 3] annulation reactions
In the meantime, the catalytic asymmetric [3 + 3] annulation reaction of β,γ-unsaturated α-ketoesters has received great attention in recent years, and a number of methodologies have been developed for synthesis of various of six-member carbo- or heterocyclic compounds. In 2015, the Lopp group exhibited the feasibility of organo-catalyzed cascade Michael addition-cyclization reaction of β,γ-unsaturated α-ketoesters with enols 90-a (scheme 13A) (Lopp et al., 2015). The adducts 91 bearing a chiral hemiketal unit were created as a single diastereoisomer in excellent yields (42–93%) and enantioselectivities (87–96% ee). In 2016, one-pot synthesis of optically active hydroquinoline-2-carboxylates 93 was described by Shi et al. (Scheme 13B) (Rong et al., 2016). This chiral base catalyzed [3 + 3] process was enabled by a sequential asymmetric Michael/transamination/cyclization process. The desired products were obtained from 1,3-cyclohexanediones, β,γ-unsaturated α-ketoesters, and benzylamine with good diastereoselectivity and high enantioselectivity (51–98% ee, 5:1 to 11:1 dr). Recently, Kowalczyk et al. applied this [3 + 3] strategy to the catalytic asymmetric desymmetrization of prochiral cyclic 1,3-diketones 92 (Scheme 13C) (Dajek et al., 2020). The unprecedented desymmetrization of the parent C- and O-centered dinucleophiles led to hemiacetals 94 with high stereoselectivities (91–99% ee), which were readily transformed to 1,4-dihydropyridines without erosion of optical purity. Moreover, other dinucleophile analogues, such as enols 90b-d (Kostenko et al., 2018; Liu et al., 2019; Modrocka et al., 2018; Pratap Reddy Gajulapalli et al., 2016; Tukhvatshin et al., 2019; Yin et al., 2016), as well as pyrazolones 95 (Kumarswamyreddy and Kesavan, 2016) and 1,3-cyclohexanediones 92 (Dajek et al., 2018; Yang et al., 2020), which could easily be tautomerized to its enol form, served as suitable C3 synthons in catalytic asymmetric [3 + 3] annulation reactions with β,γ-unsaturated α-ketoesters.
Scheme 13.
Catalytic asymmetric [3 + 3] annulation reactions with dinucleophile analogues
Moreover, in consideration of the facile deprotonation at both α-positions, aliphatic ketones could be employed as alternative dinucleophiles for C3 synthons for [3 + 3] annulation reactions. In this context, a catalytic asymmetric Michael addition-proton transfer-aldol reaction cascade reaction of α-purine-substituted acetones 96 with β,γ-unsaturated α-ketoesters was accomplished by Niu and Guo et al., in 2018 (scheme 13D) (Huang et al., 2018). By using bifunctional thiourea catalysis, chiral six-membered carbocyclic purine nucleoside analogues 97 bearing three chiral stereocenters including a chiral tetrasubstituted carbon center were readily created in high yields (65–89%) with excellent diastereo- and enantioselectivities (92–98% ee, 80:20 to 90:10 dr). Interestingly, a weak base as an additive was necessary to enhance the yields. Later, the asymmetric [3 + 3] annulation reactions of β,γ-unsaturated α-ketoesters and cyclohexanones for the synthesis of bridged compounds were reported by the Juaristi group (Cruz-Hernández et al., 2018; Obregón-Zúñiga et al., 2017). Because pyrazolones were frequently found as an important structural unit in a variety of valuable nitrogen heterocycles, α,β-unsaturated pyrazolones possessing a γ-hydrogen have been employed as nucleophiles in organic synthesis. In 2019, Feng et al. achieved an asymmetry [3 + 3] annulation reaction between β,γ-unsaturated α-ketoesters and α-arylidene pyrazolinones 98 via a Michael−aldol domino process (scheme 13E) (Xu et al., 2019b). Chiral N,N′-dioxide-Sc(III) complex displayed excellent catalytic capability under aqueous media, affording a series of spirocyclohexene pyrazolones 99 with three stereocenters including vicinal tetrasubstituted stereocenters in good to high yields (60–99%) with excellent ee values (58–94% ee). It is noteworthy that spirocyclic quaternary carbon centers could be epimerizated because of the retro-aldol process even in the absence of bases. Besides, organocatalysts such as quinidine (Krishna et al., 2020) and 9-Amino-(9-deoxy)epi-quinine-based thiourea (Sun et al., 2020) were also applicable to this kind of asymmetric process.
Besides, as another type of important reactive intermediate, enamine has a very extensive application in organocatalysis. In 2019, the Liu group developed a [3 + 3] annulation reactions of β,γ-unsaturated α-ketoesters with chroman-2-ols 100 via an enamine-catalyzed sequential process (scheme 14) (Lv et al., 2019). Fused polycyclic hemiketal derivatives trans-101 bearing three stereocenters were obtained in high yields (57–73%) with excellent diastereo- and enantioselectivities (94–99% ee, >20:1 dr). By simply treatment with boron trifluoride etherate, the enantiomers cis-101 were prepared in a highly stereoselective fashion via dehydration-condensation. It is noteworthy that the catalytic system retained high catalytic and stereocontrolling abilities after multiple recycle.
Scheme 14.
Catalytic asymmetric [3 + 3] annulation reactions involving enamine intermediate
Notably, electron-rich aromatics could be utilized as right partners in catalytic asymmetric [3 + 3] annulation reactions of β,γ-unsaturated α-ketoesters. Out of them, 2-naphthols have attracted much attention as versatile electron donor in various addition and oxidation reactions at 1-possition. A organo-catalyzed asymmetric [3 + 3] annulation of β,γ-unsaturated α-ketoesters 1b with 2-naphthols 102 was established by the Kesavan group in 2016 (scheme 15A) (Muthusamy et al., 2016). The protocol showcased a highly enantioselective synthetic strategy to construct spirooxindole-naphthopyran scaffold via a Friedel-Crafts type/hemiketalization sequential process followed by a dehydration by treating with sulfuric acid. Subsequently, Liu and Feng et al. expanded the [3 + 3] annulation of β,γ-unsaturated α-ketoesters to electron-rich phenols, using their developed N,N′-dioxide scandium (III) catalytic system (scheme 15B) (Hao et al., 2017). Excellent yields (71–97%) and high enantioselectivities with high diastereoselectivities (80–95% ee, 6:1 to 14:1 dr) were achieved under mild conditions. Interestingly, probably owing to the high reactivity and steric resistance, substrates containing N,N′-dimethylamino failed to produce the corresponding chiral product even with a high yield. In addition, pyrrole and indole derivatives served as suitable 1,3-dinucleophiles, in light of their biological significance. In 2018, the Wang group described a metal and organo co-catalyzed sequential Friedel-Crafts/aldol tandem reaction of isatin-derived β,γ-unsaturated α-ketoesters 1b and 3-pyrrolyloxindoles 106 (scheme 15C) (Li et al., 2018a).81 Bispirooxindoles 107 and diastereomeric (iso)-107 (not shown) were separately prepared in high yields (62–92%) with excellent diastereo- and enantioselectivities (81–97% ee, 90:1 to >20:1 dr).
Scheme 15.
Catalytic asymmetric [3 + 3] annulation reactions with electron-rich aromatics
For tackling the influences of the coordinative groups in asymmetric catalysis, the Tang group is devoted to developing a side arm strategy for chiral ligand design. In 2018, Wang and Tang et al. described a Ni(II)/L22 catalyzed asymmetric cascade annulation of β,γ-unsaturated α-ketoesters with electron-rich phenols (scheme 15D) (Ren et al., 2018). A broad range of substrates containing amino or methoxy groups were obtained in high yields (87–98%) and excellent enantioselectivities (89–99% ee). Noteworthily, the highly efficient synthesis of potential anticancer agents highlighted the synthetic utility of this method in preparation of chroman-based medicines.
Later, in 2020, one pot synthesis of tetrahydroindolizines 111 in three steps from β,γ-unsaturated α-ketoesters and 2-(pyrrol-1-yl)acetic acids 110 was reported by the Smith group (scheme 15E) (Zhang et al., 2020). The proposed reaction progress involved an enantioselective Michael lactonization of 2-(pyrrol-1-yl)acetic acids with β,γ-unsaturated α-ketoesters, ring-opening of the cyclized intermediate int-25 and a following spontaneous cyclization of the pyrrole ring with the pendant ketone group. Recently, Antilla et al. demonstrated a catalytic asymmetric C7 Friedel-Crafts alkylation/N-hemiacetalization cascade reaction derived from β,γ-unsaturated α-ketoesters and 4-aminoindoles 112 (scheme 15F) (He et al., 2021a). The C4-alkyamine substituent as a para-directing group was necessary for the regioselective C7 Friedel-Crafts alkylation. The reactions occurred smoothly in the presence of a chiral magnesium phosphate catalyst, resulting in the polycyclic 4H-pyrrolo [3,2,1-ij]quinoline derivatives 113 in high yields (80–96%) with good to excellent ee values (76–99% ee).
Catalytic asymmetric [4 + n] annulation reactions
Catalytic asymmetric [4 + 2] annulation reactions
Six-membered rings are one of the most common motifs in natural products, pharmaceuticals as well as functional materials. Catalytic asymmetric [4 + 2] annulation reactions represent the most direct and convenient strategy to such six-membered rings including carbo- and hetero-cycles. For example, chiral 1,4-dihydropyridines are a class of important heterocycles found in numerous natural and non-natural products, such as alkaloids, calcium channel blockers and drugs. The development of the synthetic methodology for chiral 1,4-dihydropyridines is of considerable interest. In this regard, asymmetric [4 + 2] annulations involving β,γ-unsaturated α-iminoester proved to be an efficient approach. In 2015, Zheng and Zhang et al. reported an asymmetric Michael addition-cyclocondensation reaction derived from β,γ-unsaturated α-ketoesters, arylamines and 1,3-dicarbonyl compounds (scheme 16A) (An et al., 2015). In the optimization of the conditions, a H8-BINOL type chiral imidodiphosphoric acid showed high catalytic and stereocontrolling abilities to offer a series of 1,4-dihydropyridines 116 in moderate yields (34–61%) with excellent ee values (75–97% ee). The proposed reaction progress involved a dehydration condensation of β,γ-unsaturated α-ketoesters and arylamines to produce β,γ-unsaturated α-iminoesters, the following Michael addition with 1,3-dicarbonyl compounds, and the final intramolecular dehydration condensation. Independently, a similar enantioselective three-component aza-Diels−Alder reaction of β,γ-unsaturated α-ketoesters, cyclic ketones and arylamines was developed by the Wang group using a chiral bimetallic catalysis (Deng et al., 2015).
Scheme 16.
. Catalytic asymmetric [4 + 2] annulation reactions via Michael addition-cyclocondensation
In addition, without the assistance of amines, [4 + 2] annulations between β,γ-unsaturated α-ketoesters and aldehydes were viable. In 2016, a proline-derived thiourea catalyzed asymmetry [4 + 2] cycloaddition of β,γ-unsaturated α-ketoesters and cyclic β-oxoaldehydes was reported by the Kesavan group (scheme 16B) (Vishwanath et al., 2016). The transformation was supposed to start with a 1,4-Michael addition of β,γ-unsaturated α-ketoesters, followed by a hemiacetalization cyclization. Dihydropyran-oxindole architectures containing three contiguous chiral centers were created in an efficient and highly stereoselective fashion. Subsequently, the Headley group reported a diarylprolinol silyl ethers catalyzed enantioselective [4 + 2] annulation of β,γ-unsaturated α-ketoesters and aldehydes (scheme 16C) (Katakam et al., 2017). The proposed reaction mechanism involved generation of enamines from aldehydes and secondary amine catalysis, 1,4-addition to the β,γ-unsaturated α-ketoesters, cyclocondensation and the final hydrolysis. trans-2-Oxo-3,4-dihydro-2H-pyran derivatives 120 were obtained by simple oxidation of PCC in high yields (51–94%) with excellent diastereo- and enantioselectivities (93–98% ee). Differently, Kano and Maruoka et al. reported that cis-dihydropyrans derivatives 121 were prepared as dominant product (scheme 16D) (Kano et al., 2018). The hydrogen bonding between the amino diol catalyst and substrate could stabilize the less favored transition state and lead to cis-isomers.
Besides, the Zhao group completed an asymmetric tandem inverse-electron-demand hetero-Diels–Alder/oxa-Michael reaction catalyzed by the modularly designed organocatalysts (MDOs) which were self-assembled from amino acids and cinchona alkaloid derivatives in the reaction medium (Scheme 16E) (Huang et al., 2016a). The reactions were derived from β,γ-unsaturated α-ketoesters 1d and the aldehydes 122. The catalytic cycle started with formation of enamine intermediate from aldehydes and secondary amine catalysis. Hydrogen bonding between dicarbonyl of β,γ-unsaturated α-ketoesters and thiourea contributed to decreasing the LUMO energy of β,γ-unsaturated α-ketoester and promoting the inverse-electron-demand Diels-Alder reaction. Hydrolysis occurred to give the catalysis regeneration and hemiacetals int-30, and was followed by final Michael cycloaddition. Under the catalysis of different combination of amino acids and cinchona alkaloid derivatives, enantiomers of cis- and trans-fused 123 were separately obtained in high yields (70–95%) with excellent diastereo- and enantioselectivities (94–99% ee, 88:12 to 99:1 dr).
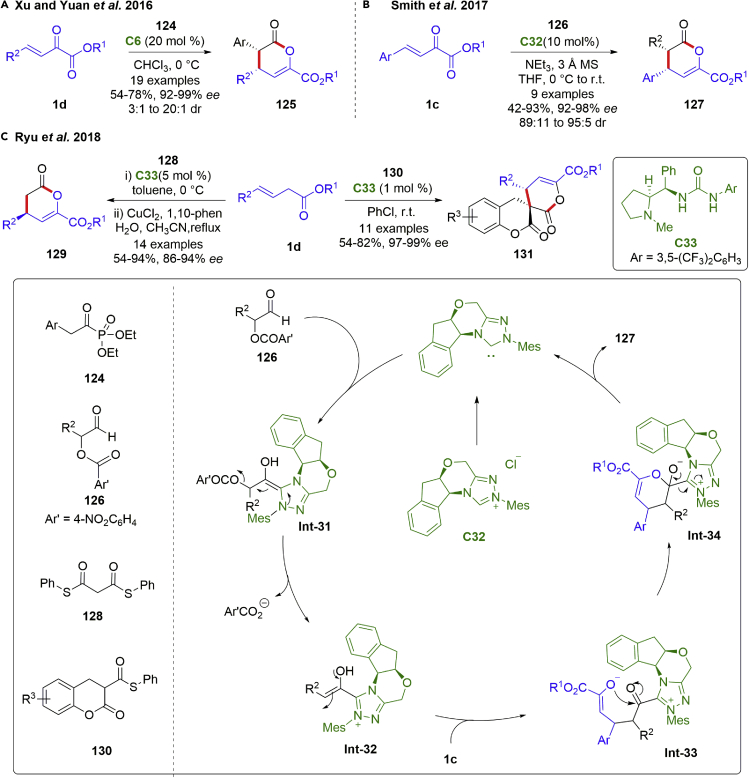
The Michael addition-lactonization process has also been employed as a practical strategy for [4 + 2] annulation reactions of β,γ-unsaturated α-ketoesters. For instance, Xu and Yuan et al. accomplished a chiral bifunctional thiourea-tertiary amine catalyzed enantioselective Michael addition-lactonization of β,γ-unsaturated α-ketoesters with arylacetyl phosphonates in 2016 (Scheme 17A) (Zhang et al., 2016). This method set up a new approach toward highly enantioenriched syn-3,4-dihydropyranone derivatives 125. Good yields (54–78%), excellent stereoselectivities (92–99% ee) and high functional group tolerance were achieved.
Scheme 17.
Catalytic symmetric [4 + 2] annulation reactions via Michael addition−lactonization
Especially, α-aroyloxyaldehydes are a class of important azolium enolate precursors, which could be employed as versatile nucleophilic reagents as well as good nucleophilic acceptors. In 2017, the Smith group developed an NHC-catalyzed enantioselective hetero-Diels−Alder reactions of β,γ-unsaturated α-ketoesters 1c and α-aroyloxyaldehydes 126 (scheme 17B) (Taylor et al., 2017). The catalytic cycle was initiated by the generation of azolium enolate int-31 via addition-eliminations. The following 1,4-addition to β,γ-unsaturated α-ketoesters and lactonization led to the adducts and NHC regeneration. Excellent diastereo- and enantioselectivities (92–98% ee, 89:11 to 95:5 dr) were achieved. The syn-dihydropyranone products bearing electron-deficient aryl were more susceptible to epimerize at the C (4)-position in the presence of base, and reduced diastereoselectivity were observed. In addition, this catalytic system was also adapted to the α,β-unsaturated γ-ketoester substrates (not shown).
Subsequently, in 2018, the Ryu group reported a proline-derived urea catalyzed enantioselective Michael addition-lactonization reactions of β,γ-unsaturated α-ketoesters and thioesters (scheme 17C) (Jin et al., 2018). Chiral 3,4-dihydropyranones 129 were obtained in high yield (54–94%) with excellent enantioselectivities (86–94% ee) via [4 + 2] annulation reactions with disulfide malonate followed by hydrolysis−decarboxylation with retention of optical purities. In addition, spiro-3,4-dihydrocoumarin-fused 3′,4′-dihydropyranones 131 were created in excellent stereoselectivities (97–99% ee) by means of this protocol using S-Phenyl-2-oxochroman-3-carbothioates 130.
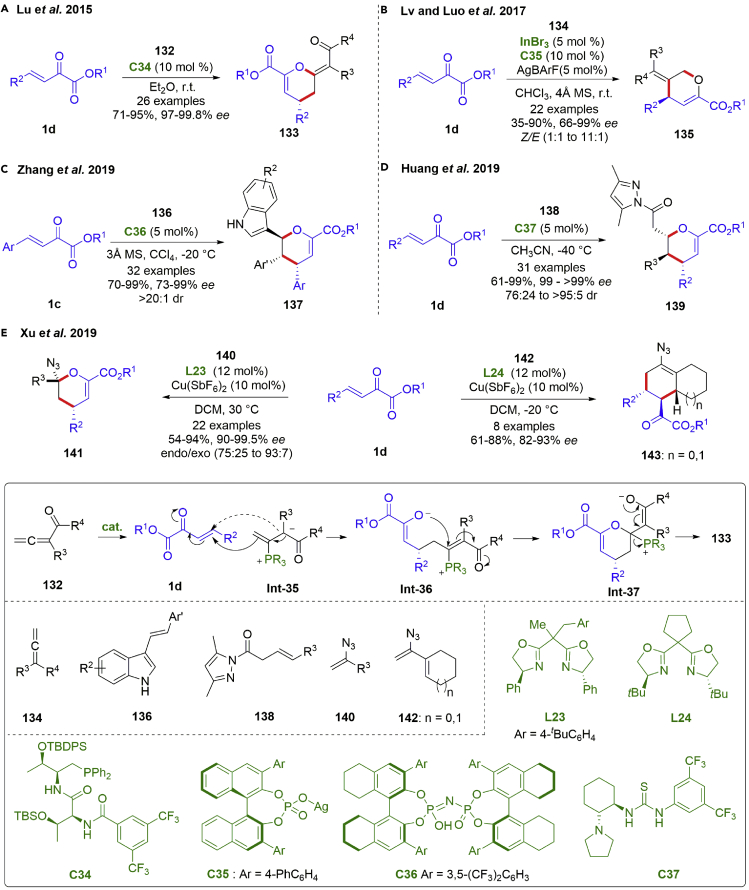
In addition to above Michael-addition type cascade annulations, inverse-electron-demand (IED) hetero-Diels–Alder reaction of β,γ-unsaturated α-ketoesters with electron rich alkenes proved to be a powerful protocol for six-member heterocycle construction. As a special compound containing a cumulative diene functionality, allene has many novel reaction performances especially in cyclization transformations. Among them, asymmetric [4 + 2] annulation reactions of β,γ-unsaturated α-ketoesters and allenes provided a powerful method for chiral six-membered heterocycles. In 2015, the Lu group developed a catalytic asymmetry [4 + 2] annulation between β,γ-unsaturated α-ketoesters and allene ketones (Scheme 18A) (Yao et al., 2015). This transformation proceeded smoothly with catalysis of dipeptide-based bifunctional phosphines, producing enantiomerically enriched 3,4-dihydropyrans 133 in high yields (71–95%) with excellent enantioselectivities (97-99.8% ee). The envisaged progress started with a nucleophilic addition of phosphine catalyst at the β-position of α-substituted allene ketone to produce α-anion Int-35. Isomerization encountered to form the γ-anion followed by an 1,4 addition to the β,γ-unsaturated α-ketoesters. The presence of α-alkyl group of the allene was crucial, which could suppress [3 + 2] cycloaddition by preventing enolate C-attack at the carbonyl α-position. The enolate O-attack at the β-position led to the formation of six-member heterocycle. In spite of the achievements with allene ketones, unactivated allenes involved catalytic enantioselective [4 + 2] annulation remains challenging. The elegant work came from the Luo group, by employing their developed cationic indium (III)/phosphate strategy. The [4 + 2] annulation of β,γ-unsaturated α-ketoesters and non-activated allenes readily occurred to prepare the corresponding C3-selective dihydropyrans 135 in good yields (35–90%) with high enantioselectivities (66–99% ee) (Scheme 18B) (Wang et al., 2017). Later, they found the electron-rich alkoxyallenes were also tolerated (Li et al., 2018b).
Scheme 18.
Catalytic asymmetric inverse-electron-demand hetero-Diels–Alder reactions
Then, in 2019, the Zhang group reported an imidodiphosphoric acid catalyzed inverse-electron-demand hetero-Diels–Alder reaction of β,γ-unsaturated α-ketoesters 1c and 3-vinylindoles 136 (scheme 18C) (Guan et al., 2019). 3,4-Dihydro-2H-pyran structural motifs bearing three contiguous stereogenic centers were created highly efficiently with excellent diastereo- and enantioselectivities (73–99% ee, >20:1 dr). Double hydrogen bond between catalyst and two reactants accounted for the enantio-control. In the same year, a dienolate-mediated asymmetric IED hetero-Diels–Alder reaction of β,γ-unsaturated α-ketoesters and β,γ-unsaturated amides was established by the Huang group (scheme 18D) (Qin et al., 2019). High yields (61–99%), excellent diastereo- and enantioselectivities (99->99% ee, 76:24 to >95:5 dr) were achieved under the catalysis of chiral thiourea. Similarly, a chiral bifunctional squaramide catalyst was utilized in the catalytic asymmetric inverse-electron-demand hetero-Diels–Alder of isatin-derived β,γ-unsaturated α-keto esters with allyl ketones by the Du group in 2020 (Lin et al., 2020). In addition, Zhang and Shi et al. described a Lewis acid-catalyzed [4 + 2] hetero-Diels–Alder reaction of 3-alkyl-2-vinylindoles with β,γ-unsaturated α-ketoesters recently (Sun et al., 2022).
It is worth noting that organic azides have attracted extensive attention over the last few decades, many of which exhibit biological activity and are employed as versatile organic synthons in diverse transformations. In 2019, the Xu groups developed a copper (II)/BOX-catalyzed IED hetero-Diels–Alder reactions of β,γ-unsaturated α-ketoesters with vinyl azides (scheme 18E) (Thirupathi et al., 2019). A broad range of optically active cyclic azides 141 were obtained in high yields (54–94%) with excellent enantioselectivities (90-99.5% ee). In contrast, cyclohexenyl azide 142 served as dienophiles and led to normal Diels-Alder reactions.
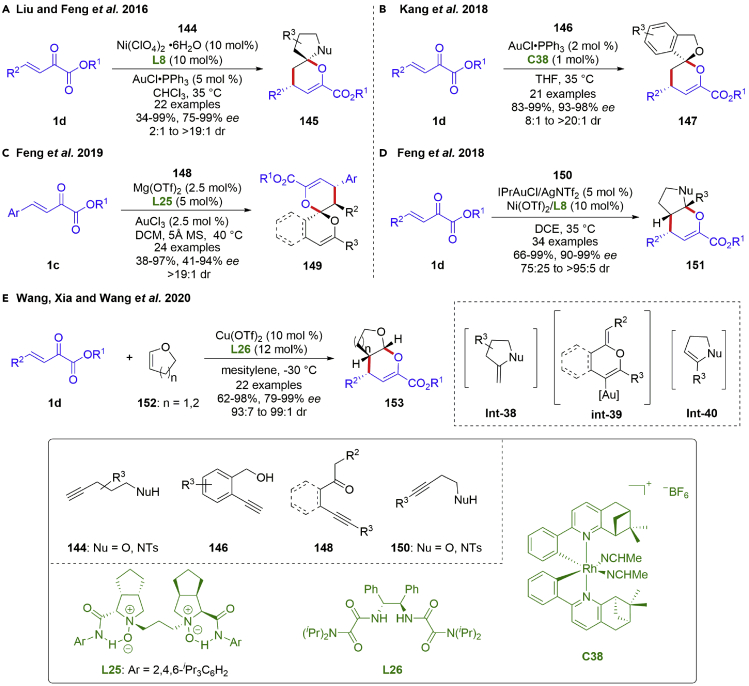
Alkynyl alcohols and amides could undergo intramolecular cyclization to form enol ethers, which are versatile activated C2-synthons, participating in diverse transformations especially annulation reactions. In 2016, Liu and Feng et al. demonstrated an asymmetric [4 + 2] annulation reactions of β,γ-unsaturated α-ketoesters with alkynyl alcohols and amides (scheme 19A) (Li et al., 2016a). The transformation proceeded smoothly with catalysis of a bimetallic gold(I)/chiral N,N′-dioxide nickel (II) complex. The corresponding spiroketals and spiroaminals 145 rather than fused bicyclo products were obtained in good yields (34–99%) with excellent diastereo- and enantioselectivities (75–99% ee, 2:1 to >19:1 dr). In addition, gold(I)/chiral Rh(III) Lewis acid was utilized as an effective catalytic system in [4 + 2] annulation reactions of β,γ-unsaturated α-ketoesters with alkynyl alcohols by the Kang group in 2018 (scheme 19B) (Gong et al., 2018). Good diastereo- and excellent enantioselectivities (93–98% ee, 8:1 to >20:1 dr) were achieved owing to the superior asymmetric induction of newly designed chiral Rh(III) complex with steric bulk around rhodium center. β-Alkynyl ketones have received great attention as fascinating precursors for construction of biologically interesting cyclic units. In 2019, the Feng group reported an asymmetric bimetallic gold (III) and chiral N,N′-dioxide/Mg(II) catalyzed cascade cycloisomerization/[4 + 2] annulation of β,γ-unsaturated α-ketoesters with β-Alkynyl ketones (scheme 19C) (Ge et al., 2019). The proposed reaction mechanism involved Au(III)-catalyzed 6-endo-dig oxo-cyclization of β-Alkynyl ketones to generate key gold-bound alkene int-39, and final Mg(II)-catalyzed inverse-electron-demand hetero-Diels–Alder reactions. 6,6-Spiroketals 149 baering three contiguous stereocenters were prepared in high yields (38–97%) with excellent diastereo- and enantioselectivities (41–94% ee, >19:1 dr).
Scheme 19.
Catalytic asymmetric [4 + 2] annulation reactions with enol ethers.
On the other hand, because the endo-enol ether intermediate is more stable than the exo-isomer, it is credible for the isomerization of exo-enol ether intermediate into the endo-isomer, which could undergo [4 + 2] annulation reactions with β,γ-unsaturated α-ketoesters to prepare the corresponding fused bicyclic products. In 2018, Feng et al. completed an asymmetric bimetallic gold(I) and chiral N,N′-dioxide/Ni(II) catalyzed cascade cyclization/inverse-electron-demand hetero Diels-Alder reaction to prepare a series of fused bicyclic N,O-acetals and O,O-acetals 151 containing a chiral tetrasubstituted carbon center in moderate to excellent yields (66–99%) with excellent diastereo- and enantioselectivities (90–99% ee, 75:25 to >95:5 dr) (Scheme 19D) (Hu et al., 2018). It is noteworthy that the yield of the fused bicyclic product could be further increased by addition of AgNTf2, indicating the electron-deficient gold(I) catalysis was favored for the transformation. Besides, [4 + 2] annulation reactions of β,γ-unsaturated α-ketoesters with preformed enol ether had been established by Wang, Xia and Wang et al., utilizing their newly developed C2-symmetric chiral bis-oxalamides/Cu(II) complex as catalyst (scheme 19E) (Chen et al., 2020). The authors highlighted the carbonyl coordination of the chiral ligand to Cu(II) contributing to superior asymmetric induction.
Catalytic asymmetric [4 + 1] and [4 + 3] annulation reactions
In contrast to the well-developed [4 + 2] annulations, catalytic asymmetric [4 + 1] and [4 + 3] annulation reactions of β,γ-unsaturated α-ketoesters received less attention despite the biological and synthetic importance of the corresponding five- and seven-membered heterocycles. For example, 2,3-dihydrofuran scaffolds are present in numbers of important molecules, many of which have been reported to exhibit interesting biological activities. Asymmetric [4 + 1] annulation reactions involving β,γ-unsaturated α-ketoesters provided an available tool for construction of these five-membered heterocycles. In 2017, the Li group reported a chiral phosphine-catalyzed enantioselective [4 + 1] annulation reactions between β,γ-unsaturated α-ketoesters and Morita−Baylis−Hillman carbonates to prepare a series of 2,3-dihydrofurans 155 in moderate to high yields (22–82%) with excellent asymmetric induction (94–99% ee, >20:1 dr) (Scheme 20A) (Cheng et al., 2017). This presented catalytic system was also adapted to other electron-deficient olefins.
Scheme 20.
Catalytic asymmetric [4 + 1] and [4 + 3] annulation reactions
Furthermore, tandem cyclization or cycloaddition reactions provide a powerful method for convenient access to polycyclic compounds. In 2019, Liu and Feng et al. reported a catalytic asymmetric cascade reaction of β,γ-unsaturated α-ketoesters and diazoimides (scheme 20B) (Xu et al., 2019a). The reaction’s progress involved a [4 + 3] annulation of β,γ-unsaturated α-ketoesters and isomünchnones Int-41 generated in situ from diazoimides. Bimetallic relay catalysis of rhodium (II) and chiral N,N′-dioxide-zinc(II) complex under mild conditions facilitated the synthesis of structurally sophisticated oxa-bridged oxazocine products 157 in moderate to excellent yields (51–90%) and excellent ee values (85–95% ee).
Miscellaneous reactions
Polycyclic compounds bearing fused ring, bridge ring or spiro ring have occupied a central role in the annals of the natural products, fine chemicals, ligands or catalysis as well as other complex molecules, and still pose a great challenge to organic synthesis. It is an appealing strategy to construct polycyclic skeletons straightforwardly by taking advantage of the multiple reactive sites of β,γ-unsaturated α-ketoesters in reaction cascades.
For instance, Zeng and Zhong et al. developed a modified β,γ-unsaturated α-ketoesters 1l bearing nucleophilic NH group on the phenyl ring. In 2017, L-proline-derived secondary amine catalyzed asymmetric cascade aza-Michael-IED/HAD reaction of 1l with enals 158 was achieved (scheme 21A) (Dai et al., 2017). A variety of tetrahydropyranoquinoline derivatives 159 with four stereogenic centers were prepared conveniently in moderate to good yields (56–88%) with excellent diastereo- and enantioselectivities (97–99% ee, 89:11 to 99:1 dr). The crystallographic analysis, NMR studies in situ and isolation of the key intermediate Int-42 indicated the reaction mechanism involved the aza-Michael addition between sulfonamide and an activated iminium ion derived from enals and secondary amine, the intramolecular [4 + 2] annulation and the final substitution of H2O to give the desired products and secondary amine catalysis regeneration.
Scheme 21.
Catalytic asymmetric cascade reaction for synthesis of polycyclic compounds
By using a similar modified β,γ-unsaturated α-ketoesters 1m, Qiao and Wang et al. reported a Takemoto thiourea catalyst and triflimide cooperatively catalyzed Michael/hemiketalization/Pictet−Spengler cyclization with 3-pyrrolyloxindoles 108 for the construction of bridge-ring skeletons (scheme 21C) (Fan et al., 2017). The projected reaction was initiated by organo-catalyzed Michael addition, which was the enantio-determining step. The following intramolecular hemiketalization resulted in Int-44. The final Pictet−Spengler cyclization was promoted by a Brønsted acid. One tetrasubstituted spiro stereogenic carbon center and two bridgehead carbon centers were created with excellent diastereoselectivities and enantioselectivities (73–99% ee, 4:1 to >20:1 dr). In a follow-up research, the reaction cascade of β,γ-unsaturated α-ketoesters 1n with pyrazolones 164 was set up by the same group via a Michael/ketalization process (scheme 21E) (Fan et al., 2020). The transformation was conducted by a chiral binaphthyl box-copper catalysis, and afforded a series of functionalized bicyclononane skeletons 165 in high yields (39–85%) with excellent enantio- and diastereoselectivities (66–97% ee, >20:1 dr).
In 2017, the Wang groups accomplished an NHC catalyzed asymmetric Michael-intramolecular aldol-lactonization cascade for spirocyclic oxindole skeletons construction (scheme 21B) (Zhang et al., 2017). The transformation proceeded the generation of Breslow homoenolate intermediate Int-43 by addition of the chiral aminoindanol-derived triazolium-based NHC to enals. The subsequent [3 + 2] annulation reaction was initiated by Michael addition, and the final intramolecular acylation reaction accounted for the lactone formation and NHC regeneration. The desired β-propiolactone-fused spiro [cyclopentane-oxindoles] 160 bearing four contiguous stereocenters including a spiro all-carbon quaternary stereogenic center were yielded with excellent diastereo- and enantioselectivities (73–99% ee, >99:1 dr).
Besides, in 2019, one pot synthesis of chromenopyrrolidines 163 containing four contiguous stereocenters was established by Xu et al. (scheme 21D) (Cao et al., 2019). The chemical event included enantioselective [2 + 3] annulation/hemiketalization reaction cascade between β,γ-unsaturated α-ketoesters and o-hydroxy aromatic aldimines 162 and the following acylation. The desired products were obtained in high yields with excellent stereoselectivities under the catalysis of a chiral bifunctional thiourea-tertiary amine. Similarly, the Li group demonstrated that isotetronic acid-fused thiochromanes 167 could be prepared via the sulfa-Michael/aldol/lactonization cascade in a highly stereoselective fashion (Scheme 21F) (Mo et al., 2021). The authors highlighted the lactonization of a less-explored ester carbonyl group of β,γ-unsaturated α-ketoesters.
Conclusions
β,γ-Unsaturated α-ketoesters have become versatile organic synthons participating in diverse catalytic asymmetric transformations with the breathtaking development of organo-catalysis, new catalytic system including ingenious chiral ligands as well as Lewis acid cation. This review summarized the recent developments of β,γ-unsaturated α-ketoesters according to the below reaction modes: (1) thanks to different characteristics of the catalytic system, the regioselective 1,4-and 1,2-addtions with diversified nucleophiles reveal straightforward and powerful protocols for enantio-enriched ketoesters and tertiary alcohols, (2) multiform cyclization reactions including [2 + 3], [2 + 4], [3 + 2], [3 + 3] and [4 + 2] annulation afford efficient and convenient approaches to five- and six-membered ring skeletons, (3) especially we highlighted the utilization of β,γ-Unsaturated α-ketoesters in construction of polycyclic skeletons. In general, the highly efficient creation of stereogenic centers with excellent enantioselectivities is not a surprise, but owes to the bidentate coordination of its unique 1,2-dicarbonyl motif to artful chiral messenger, establishing a rigid system for the precise chiral-identification of the attack.
In spite of the tremendous achievement achieved, persistent efforts are required for rising to some challenges remaining in this research area, including but not limited to the followings. (1) β,γ-Unsaturated α-ketoesters showed a strong partiality for C-attack according to reported literature, whereas various nucleophiles including organosilicons, sulfides, and phosphides etc. are highly desired. (2) Most research mainly focused on developing new synthetic methodologies for five or six-member carbo- and heterocycles, and there is a great prospect for applications in construction of strained small rings via [2 + 1] or [2 + 2] annulations or macrocycles via [3 + n] (n ≥ 4) or [4 + n] (n ≥ 3) annulations. (3) β,γ-Alkynyl ketoesters are among one of the important analogue but with limited investigations. (4) With manipulatable functional groups, the chiral products are important and useful synthetic intermediates. There is tremendous potential waiting to be excavated for application in natural product synthesis, pharmaceutical chemistry, material science and other fields. The authors believe that these targets will be realized with the continuous endeavors of chemists in exploiting new reaction modes and catalytic systems. The next decades will witness a flourishing and prosperous panorama in this exciting area of research.
Acknowledgments
Financial support from the Scientific Research Starting Foundation of Zhengzhou University, application research plan of key scientific research projects in Colleges and universities of Henan Province (22A150056), and China Postdoctoral Science Foundation (2021TQ0290) are gratefully acknowledged.
Author contributions
T.-J.H., X. G., Y.-F.Y., and G.-J. M. collected the references. R.D. and G.-J.M. conceived the project and prepared this manuscript. G.-J.M. overall supervised the project.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Ruixian Deng, Email: rxdeng@zzu.edu.cn.
Guang-Jian Mei, Email: meigj@zzu.edu.cn.
References
- An D., Zhu Z., Zhang G., Gao Y., Gao J., Han X., Zheng L., Zhang S. H8-BINOL chiral imidodiphosphoric acids catalyzed cyclization reactions of β,γ-unsaturated α-ketoesters, arylamines and 1,3-dicarbonyl compounds: enantioselective synthesis of 1,4-dihydropyridines. Tetrahedron. 2015;26:897–906. doi: 10.1016/j.tetasy.2015.06.019. [DOI] [Google Scholar]
- Bai M., Chen Y.-Z., Cui B.-D., Xu X.-Y., Yuan W.-C. Thiourea-catalyzed asymmetric domino Michael-cyclization reaction of 3-isothiocyanato oxindoles with β,γ-unsaturated α-keto esters for the synthesis of spirocyclic oxindoles. Tetrahedron. 2019;75:2155–2161. doi: 10.1016/j.tet.2019.02.036. [DOI] [Google Scholar]
- Cao J., Liu J.-Y., Zhang Y.-M., Wang Z.-Y., Xu P.-F. Synergistic promotion by intramolecular hydrogen bonding: a bi-functionally catalyzed cascade reaction for the synthesis of enantiopure chromenopyrrolidines. Org. Chem. Front. 2019;6:674–678. doi: 10.1039/c8qo01208c. [DOI] [Google Scholar]
- Chen J.-B., Xu M., Zhang J.-Q., Sun B.-B., Hu J.-M., Yu J.-Q., Wang X.-W., Xia Y., Wang Z. Modular Chiral bisoxalamide–copper-catalyzed asymmetric Oxo-Diels–Alder reaction: carbonyl coordination for high enantio- and diastereocontrols. ACS Catal. 2020;10:3556–3563. doi: 10.1021/acscatal.9b05606. [DOI] [Google Scholar]
- Chen S., Wang Y., Zhou Z. Organocatalyzed asymmetric Michael addition of 1-acetylindolin-3-ones to β,γ-unsaturated α-ketoesters: an access to chiral indolin-3-ones with two adjacent tertiary stereogenic centers. J. Org. Chem. 2016;81:11432–11438. doi: 10.1021/acs.joc.6b02070. [DOI] [PubMed] [Google Scholar]
- Chen X., Jiang H., Hou B., Gong W., Liu Y., Cui Y. Boosting chemical stability, catalytic activity, and enantioselectivity of metal-organic frameworks for batch and flow reactions. J. Am. Chem. Soc. 2017;139:13476–13482. doi: 10.1021/jacs.7b06459. [DOI] [PubMed] [Google Scholar]
- Chen Y., Cui B.-D., Bai M., Han W.-Y., Wan N.-W., Chen Y.-Z. Synthesis of chiral spiro-cyclopentene/cyclopentadiene-oxindoles through an asymmetric [3 + 2] cycloaddition of isatin-derived MBH carbonates and β,γ-unsaturated α-keto esters. Tetrahedron. 2019;75:2971–2979. doi: 10.1016/j.tet.2019.04.040. [DOI] [Google Scholar]
- Cheng H.G., Lu L.Q., Wang T., Yang Q.Q., Liu X.P., Li Y., Deng Q.H., Chen J.R., Xiao W.J. Highly enantioselective Friedel-Crafts alkylation/N-hemiacetalization cascade reaction with indoles. Angew. Chem. Int. .Ed. 2013;52:3250–3254. doi: 10.1002/anie.201209998. [DOI] [PubMed] [Google Scholar]
- Cheng Y., An J., Lu L.Q., Luo L., Wang Z.Y., Chen J.R., Xiao W.J. Asymmetric cyclopropanation of β,γ-unsaturated α-ketoesters with stabilized sulfur ylides catalyzed by C2-symmetric ureas. J. Org. Chem. 2011;76:281–284. doi: 10.1021/jo101699r. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Han Y., Li P. Organocatalytic enantioselective [1 + 4] annulation of morita-baylis-hillman carbonates with electron-deficient olefins: access to chiral 2,3-dihydrofuran derivatives. Org. Lett. 2017;19:4774–4777. doi: 10.1021/acs.orglett.7b02144. [DOI] [PubMed] [Google Scholar]
- Cruz-Hernández C., Martínez-Martínez E., Hernández-González P.E., Juaristi E. Synthesis of a new n-diaminophosphoryl-n′-[(2s)-2-pyrrolidinylmethyl]thiourea as a chiral organocatalyst for the stereoselective michael addition of cyclohexanone to nitrostyrenes and chalcones - application in cascade processes for the synthesis of polyc. E. J. Org. Chem. 2018;2018:6890–6900. doi: 10.1002/ejoc.201801339. [DOI] [Google Scholar]
- Dai L., Hou Y., Zhang L., Chen Z., Zeng X., Zhong G. Construction of tetrahydropyranoquinoline derivativesviaan asymmetric organocatalytic aza-Michael-IED/HAD cascade reaction. Org. Biomol. Chem. 2017;15:9630–9637. doi: 10.1039/c7ob02231j. [DOI] [PubMed] [Google Scholar]
- Dajek M., Kowalczyk R., Boratyński P.J. Trans-1,2-diaminocyclohexane-based sulfonamides as effective hydrogen-bonding organocatalysts for asymmetric Michael–hemiacetalization reaction. Catal. Sci. Techn. 2018;8:4358–4363. doi: 10.1039/c8cy01199k. [DOI] [Google Scholar]
- Dajek M., Pruszczyńska A., Konieczny K.A., Kowalczyk R. Cinchona squaramide-catalyzed intermolecular desymmetrization of 1,3-diketones leading to chiral 1,4-dihydropyridines. Adv. Synth. Catal. 2020;362:3613–3620. doi: 10.1002/adsc.202000455. [DOI] [Google Scholar]
- Deng Y., Karunaratne C.V., Csatary E., Tierney D.L., Wheeler K., Wang H. Chiral bimetallic catalysts derived from chiral metal phosphates: enantioselective three-component asymmetric aza-diels-alder reactions of cyclic ketones. J. Org. Chem. 2015;80:7984–7993. doi: 10.1021/acs.joc.5b00895. [DOI] [PubMed] [Google Scholar]
- Desimoni G., Faita G., Quadrelli P. Substituted (E)-2-oxo-3-butenoates: reagents for every enantioselectively-catalyzed reaction. Chem. Rev. 2013;113:5924–5988. doi: 10.1021/cr4000732. [DOI] [PubMed] [Google Scholar]
- Desyatkin V.G., Anokhin M.V., Rodionov V.O., Beletskaya I.P. Polystyrene-supported Cu(II)-R-Box as recyclable catalyst in asymmetric Friedel–Crafts reaction. Russ. J. Org. Chem. 2017;52:1717–1727. doi: 10.1134/s1070428016120010. [DOI] [Google Scholar]
- Ding P.G., Hu X.S., Yu J.S., Zhou J. Diastereodivergent Synthesis of α-chiral tertiary azides through catalytic asymmetric Michael addition. Org. Lett. 2020;22:8578–8583. doi: 10.1021/acs.orglett.0c03178. [DOI] [PubMed] [Google Scholar]
- Duan C., Yao Y., Ye L., Shi Z., Zhao Z., Li X. Highly stereoselective construction of tetrahydroquinolines via cascade aza-Michael-Michael reaction: formal [4+2] cycloaddition of β,γ-unsaturated α-ketoesters with 2-aminochalcones. Tetrahedron. 2018;74:7179–7185. doi: 10.1016/j.tet.2018.10.053. [DOI] [Google Scholar]
- Eftekhari-Sis B., Zirak M. Chemistry of α-oxoesters: a powerful tool for the synthesis of heterocycles. Chem. Rev. 2015;115:151–264. doi: 10.1021/cr5004216. [DOI] [PubMed] [Google Scholar]
- Fan W.T., Li N.K., Xu L., Qiao C., Wang X.W. Organo-catalyzed asymmetric Michael-Hemiketalization-Oxa-Pictet-Spengler cyclization for bridged and spiro heterocyclic skeletons: oxocarbenium ion as a key intermediate. Org. Lett. 2017;19:6626–6629. doi: 10.1021/acs.orglett.7b03341. [DOI] [PubMed] [Google Scholar]
- Fan W.T., Yang X.P., Lv H.P., Wang X.W., Wang Z. Chiral binaphthyl box-copper-catalyzed enantioselective tandem Michael-ketalization annulations for optically active aryl and heteroaryl fused bicyclicnonanes. Org. Lett. 2020;22:3936–3941. doi: 10.1021/acs.orglett.0c01221. [DOI] [PubMed] [Google Scholar]
- Fofana M., Dudognon Y., Bertrand L., Constantieux T., Rodriguez J., Ndiaye I., Bonne D., Bugaut X. Enantioselective organocatalyzed Michael additions of nitroalkanes to 4-arylidenedihydrofuran-2,3-diones and 4-arylidenepyrrolidine-2,3-diones. E. J. Org. Chem. 2020;2020:3486–3490. doi: 10.1002/ejoc.202000460. [DOI] [Google Scholar]
- Gao T.-P., Liu D., Lin J.-B., Hu X.-Q., Wang Z.-Y., Xu P.-F. Direct construction of chiral quaternary dihydropyranones through highly enantioselective organocatalytic hetero-Diels–Alder reactions of olefinic azlactones. Org. Chem. Front. 2016;3:598–602. doi: 10.1039/c6qo00018e. [DOI] [Google Scholar]
- Ge S., Cao W., Kang T., Hu B., Zhang H., Su Z., Liu X., Feng X. Bimetallic catalytic asymmetric tandem reaction of β-alkynyl ketones to synthesize 6,6-spiroketals. Angew. Chem. Int. .Ed. 2019;58:4017–4021. doi: 10.1002/anie.201812842. [DOI] [PubMed] [Google Scholar]
- Gong J., Wan Q., Kang Q. Gold(I)/chiral rh(iii) lewis acid relay catalysis enables asymmetric synthesis of spiroketals and spiroaminals. Adv. Synth. Catal. 2018;360:4031–4036. doi: 10.1002/adsc.201800492. [DOI] [Google Scholar]
- Guan X.-K., Liu G.-F., An D., Zhang H., Zhang S.-Q. Chiral imidodiphosphoric acid-catalyzed highly diastereo- and enantioselective synthesis of poly-substituted 3,4-dihydro-2h-pyrans: [4 + 2] cycloadditions of β,γ-unsaturated α-ketoesters and 3-vinylindoles. Org. Lett. 2019;21:5438–5442. doi: 10.1021/acs.orglett.9b01675. [DOI] [PubMed] [Google Scholar]
- Hao X., Lin L., Tan F., Ge S., Liu X., Feng X. Asymmetric synthesis of chromans via the Friedel–Crafts alkylation–hemiketalization catalysed by an N,N′-dioxide scandium(iii) complex. Org. Chem. Front. 2017;4:1647–1650. doi: 10.1039/c7qo00323d. [DOI] [Google Scholar]
- He H., Cao Y., Xu J., Antilla J.C. Catalytic asymmetric C-7 friedel-crafts alkylation/N-hemiacetalization of 4-aminoindoles. Org. Lett. 2021;23:3010–3014. doi: 10.1021/acs.orglett.1c00699. [DOI] [PubMed] [Google Scholar]
- He L., Ahmed E.M.A., Liu H., Hu X., Xiao H.P., Li J., Jiang J. Asymmetric construction of α-substituted β-hydroxy lactones via ni catalyzed decarboxylative addition reaction. J. Org. Chem. 2021;86:4825–4834. doi: 10.1021/acs.joc.0c02854. [DOI] [PubMed] [Google Scholar]
- Honda K., Mikami K. Asymmetric "Acetylenic" [3 + 2] cycloaddition of nitrones catalyzed by cationic chiral pd(ii) Lewis acid. Chem. Asian J. 2018;13:2838–2841. doi: 10.1002/asia.201801016. [DOI] [PubMed] [Google Scholar]
- Horwitz M.A., Zavesky B.P., Martinez-Alvarado J.I., Johnson J.S. Asymmetric organocatalytic reductive coupling reactions between benzylidene pyruvates and aldehydes. Org. Lett. 2016;18:36–39. doi: 10.1021/acs.orglett.5b03127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Li J., Cao W., Lin Q., Yang J., Lin L., Liu X., Feng X. Asymmetric synthesis of fused bicyclic n,o- and o,o-acetals via cascade reaction by gold(i)/n,n′-dioxide-nickel(ii) bimetallic relay catalysis. Adv. Synth. Catal. 2018;360:2831–2835. doi: 10.1002/adsc.201800576. [DOI] [Google Scholar]
- Hu Y., Li Y., Zhang S., Li C., Li L., Zha Z., Wang Z. Copper catalyzed enantioselective alkylation of pyrrole with β,γ-unsaturated α-ketoesters: application to one-pot construction of the seven-membered ring by merging a gold Catalysis. Org. Lett. 2015;17:4018–4021. doi: 10.1021/acs.orglett.5b01917. [DOI] [PubMed] [Google Scholar]
- Hua Y.Z., Liu M.M., Huang P.J., Song X., Wang M.C., Chang J.B. A new strategy for enantioselective construction of multisubstituted five-membered oxygen heterocycles via a domino michael/hemiketalization reaction. Chem. Eur. J. 2015;21:11994–11998. doi: 10.1002/chem.201501655. [DOI] [PubMed] [Google Scholar]
- Huang H., Konda S., Zhao J.C. Diastereodivergent catalysis using modularly designed organocatalysts: synthesis of both cis- and trans-fused pyrano[2,3-b]pyrans. Angew. Chem. Int. .Ed. 2016;55:2213–2216. doi: 10.1002/anie.201510134. [DOI] [PubMed] [Google Scholar]
- Huang K.X., Xie M.S., Zhang Q.Y., Niu H.Y., Qu G.R., Guo H.M. Synthesis of chiral six-membered carbocyclic purine nucleosides via organocatalytic enantioselective [3 + 3] annulation. Org. Lett. 2018;20:5398–5401. doi: 10.1021/acs.orglett.8b02309. [DOI] [PubMed] [Google Scholar]
- Huang Y., Huang R.Z., Zhao Y. Cobalt-catalyzed enantioselective vinylation of activated ketones and imines. J. Am. Chem. Soc. 2016;138:6571–6576. doi: 10.1021/jacs.6b02372. [DOI] [PubMed] [Google Scholar]
- Jaiswal M.K., Kumar R., Singh S., Jain S., Vanka K., Singh R.P. The Vinylogous michael addition of 3-alkylidene-2-oxindoles to β,γ-unsaturated α-keto esters by bifunctional cinchona alkaloids. E. J. Org. Chem. 2020;2020:5690–5694. doi: 10.1002/ejoc.202000835. [DOI] [Google Scholar]
- Jin H., Lee J., Shi H., Lee J.Y., Yoo E.J., Song C.E., Ryu D.H. Bioinspired synthesis of chiral 3,4-dihydropyranones via s-to-o acyl-transfer reactions. Org. Lett. 2018;20:1584–1588. doi: 10.1021/acs.orglett.8b00331. [DOI] [PubMed] [Google Scholar]
- Juste-Navarro V., Marqués-López E., Herrera R.P. Thiourea-catalyzed addition of indoles to aliphatic β,γ-unsaturated α-ketoesters. Asian J. Org. Chem. 2015;4:884–889. doi: 10.1002/ajoc.201500154. [DOI] [Google Scholar]
- Kano T., Maruyama H., Homma C., Maruoka K. Catalyst-controlled diastereoselectivity reversal in the formation of dihydropyrans. Chem. Commun. 2018;54:3496–3499. doi: 10.1039/C8CC01443D. [DOI] [PubMed] [Google Scholar]
- Katakam N.K., Kim Y., Headley A.D. A highly enantioselective [4 + 2] cycloaddition involving aldehydes and β,γ-unsaturated-α-keto esters. Tetrahedron. 2017;28:1591–1595. doi: 10.1016/j.tetasy.2017.09.019. [DOI] [Google Scholar]
- Kim Y., Li C.-J. Perspectives on green synthesis and catalysis. Green. Synth. Catal. 2020;1:1–11. doi: 10.1016/j.gresc.2020.06.002. [DOI] [Google Scholar]
- Konda S., Guo Q.S., Abe M., Huang H., Arman H., Zhao J.C. Organocatalyzed asymmetric aldol reactions of ketones and β,γ-unsaturated α-ketoesters and phenylglyoxal hydrates. J. Org. Chem. 2015;80:806–815. doi: 10.1021/jo502254e. [DOI] [PubMed] [Google Scholar]
- Kostenko A.A., Kucherenko A.S., Komogortsev A.N., Lichitsky B.V., Zlotin S.G. Asymmetric Michael addition between kojic acid derivatives and unsaturated ketoesters promoted by C2-symmetric organocatalysts. Org. Biomol. Chem. 2018;16:9314–9318. doi: 10.1039/c8ob02523a. [DOI] [PubMed] [Google Scholar]
- Krishna A.V., Reddy G.S., Gorachand B., Ramachary D.B. Organocatalytic asymmetric formal [3 + 3]-cycloaddition to access 2,3-diazaspiro[4.5]deca-3,6-dien-1-ones. E. J. Org. Chem. 2020;2020:6623–6628. doi: 10.1002/ejoc.202001175. [DOI] [Google Scholar]
- Kuang Y., Shen B., Dai L., Yao Q., Liu X., Lin L., Feng X. Diastereodivergent asymmetric Michael-alkylation reactions using chiral N,N'-dioxide/metal complexes. Chem. Sci. 2018;9:688–692. doi: 10.1039/c7sc02757e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamyreddy N., Kesavan V. Enantioselective synthesis of dihydrospiro[indoline-3,4'-pyrano[2,3-c]pyrazole] derivatives via Michael/Hemiketalization reaction. Org. Lett. 2016;18:1354–1357. doi: 10.1021/acs.orglett.6b00287. [DOI] [PubMed] [Google Scholar]
- Li J., Lin L., Hu B., Lian X., Wang G., Liu X., Feng X. Bimetallic gold(i)/chiral n,n'-dioxide nickel(ii) asymmetric relay catalysis: chemo- and enantioselective synthesis of spiroketals and spiroaminals. Angew. Chem. Int. .Ed. 2016;55:6075–6078. doi: 10.1002/anie.201601701. [DOI] [PubMed] [Google Scholar]
- Li K., Sun X., Li L., Zha Z., Zhang F.L., Wang Z. Stereoselective copper-catalyzed direct aldol reaction of β, γ-unsaturated α-ketoesters with Coumaran-3-Ones. Chem. Eur. J. 2021;27:581–584. doi: 10.1002/chem.202003510. [DOI] [PubMed] [Google Scholar]
- Li N.-K., Kong L.-P., Qi Z.-H., Yin S.-J., Zhang J.-Q., Wu B., Wang X.-W. Friedel-crafts reaction of indoles with isatin-derived β,γ-unsaturated α-keto esters using a binol-derived bisoxazoline (box)/copper(ii) complex as catalyst. Adv. Synth. Catal. 2016;358:3100–3112. doi: 10.1002/adsc.201600272. [DOI] [Google Scholar]
- Li N.K., Fan W.T., Zhang J.Q., Sun B.B., Chen J.B., Wang X.W. Diastereodivergent synthesis of bispirooxindoles via asymmetric Friedel-Crafts/aldol cascade reaction: co-catalyst effects on diastereoselective outcomes. Chem. Commun. 2018;54:2260–2263. doi: 10.1039/c7cc09489b. [DOI] [PubMed] [Google Scholar]
- Li N.K., Zhang J.Q., Sun B.B., Li H.Y., Wang X.W. Chiral diphosphine-palladium-catalyzed sequential asymmetric double-friedel-crafts alkylation and n-hemiketalization for spiro-polycyclic indole derivatives. Org. Lett. 2017;19:1954–1957. doi: 10.1021/acs.orglett.7b00368. [DOI] [PubMed] [Google Scholar]
- Li R., Li Z.-L., Zhou H.-Y., He Y.-H., Guan Z. Enzyme-catalyzed asymmetric construction of chiral tertiary alcohols via aldol reaction using proteinase. J. Mol. Catal. B: Enzym. 2016;126:90–98. doi: 10.1016/j.molcatb.2016.01.008. [DOI] [Google Scholar]
- Li S., Lv J., Luo S. Enantioselective indium(i)-catalyzed [4 + 2] annulation of alkoxyallenes and β,γ-unsaturated α-keto esters. Org. Chem. Front. 2018;5:1787–1791. doi: 10.1039/c8qo00319j. [DOI] [Google Scholar]
- Li Y., Huang Y., Gui Y., Sun J., Li J., Zha Z., Wang Z. Copper-catalyzed enantioselective Henry reaction of β,γ-unsaturated α-ketoesters with nitromethane in water. Org. Lett. 2017;19:6416–6419. doi: 10.1021/acs.orglett.7b03299. [DOI] [PubMed] [Google Scholar]
- Lin Y., Hou X.Q., Li B.Y., Du D.M. Organocatalytic remote asymmetric inverse-electron-demand oxa-diels-alder reaction of allyl ketones with isatin-derived unsaturated keto esters. Adv. Synth. Catal. 2020;362:5728–5735. doi: 10.1002/adsc.202001242. [DOI] [Google Scholar]
- Lin Y., Zhao B.L., Du D.M. Organocatalytic asymmetric synthesis of 3,3'-pyrrolidinyl-bispirooxindoles via michael/n-hemiketalization cascade reaction between 3-aminooxindoles and isatin-derived β,γ-unsaturated α-keto esters. J. Org. Chem. 2018;83:7741–7750. doi: 10.1021/acs.joc.8b00632. [DOI] [PubMed] [Google Scholar]
- Liu R.-l., Yan Y.-y., Zhang T., Zhang X.-j., Yan M. A practical synthesis of optically active δ-nitro-α-ketoesters and 4-cyclohexyl-proline catalyzed by chiral squamides. Tetrahedron. 2015;26:1416–1422. doi: 10.1016/j.tetasy.2015.10.009. [DOI] [Google Scholar]
- Liu S., Xu Z.H., Wang X., Zhu H.R., Wang M.C. Azetidine-derived dinuclear zinc-catalyzed asymmetric conjugate addition of bioactive heterocycles to β,γ-unsaturated α-keto esters. J. Org. Chem. 2019;84:13881–13889. doi: 10.1021/acs.joc.9b02046. [DOI] [PubMed] [Google Scholar]
- Liu X.S., Li Y., Li X. Bi(OAc)3/chiral phosphoric acid-catalyzed enantioselective 1,2- and formal 1,4-allylation reaction of β,γ-unsaturated α-ketoesters. Org. Lett. 2021;23:9128–9133. doi: 10.1021/acs.orglett.1c03453. [DOI] [PubMed] [Google Scholar]
- Lopp M., Preegel G., Ilmarinen K., Järving I., Kanger T., Pehk T. Enantioselective organocatalytic michael addition-cyclization cascade of cyclopentane-1,2-dione with substituted (E)-2-oxobut-3-enoates. Synthesis. 2015;47:3805–3812. doi: 10.1055/s-0035-1560347. [DOI] [Google Scholar]
- Luo W., Zhao J., Ji J., Lin L., Liu X., Mei H., Feng X. A catalytic asymmetric carbonyl-ene reaction of β,γ-unsaturated α-ketoesters with 5-methyleneoxazolines. Chem. Commun. 2015;51:10042–10045. doi: 10.1039/c5cc02748a. [DOI] [PubMed] [Google Scholar]
- Lv J., Zhang Q., Zhong X., Luo S. Asymmetric latent carbocation catalysis with chiral trityl phosphate. J. Am. Chem. Soc. 2015;137:15576–15583. doi: 10.1021/jacs.5b11085. [DOI] [PubMed] [Google Scholar]
- Lv X.-J., Zhao W.-W., Chen Y.-H., Wan S.-B., Liu Y.-K. Organocatalytic asymmetric synthesis of both cis- and trans-configured pyrano[2,3-b]chromenes via different dehydration pathways. Org. Chem. Front. 2019;6:1972–1976. doi: 10.1039/c9qo00366e. [DOI] [Google Scholar]
- Ma H.-L., Li J.-Q., Sun L., Hou X.-H., Zhang Z.-H., Fu B. Heteroarylidene-tethered bis(oxazoline) copper complexes catalyzed cascade reaction involving asymmetric Friedel–Crafts alkylation/N-hemiacetalization of indoles with β,γ-unsaturated α-ketoester. Tetrahedron. 2015;71:3625–3631. doi: 10.1016/j.tet.2014.12.020. [DOI] [Google Scholar]
- Mei G.J., Koay W.L., Tan C.X.A., Lu Y. Catalytic asymmetric preparation of pyrroloindolines: strategies and applications to total synthesis. Chem. Soc. Rev. 2021;50:5985–6012. doi: 10.1039/d0cs00530d. [DOI] [PubMed] [Google Scholar]
- Miyamura H., Yasukawa T., Zhu Z., Kobayashi S. Asymmetric 1,4-addition of arylboronic acids to β,γ-unsaturated α-ketoesters using heterogeneous Chiral metal nanoparticle systems. Adv. Synth. Catal. 2019;362:353–359. doi: 10.1002/adsc.201901294. [DOI] [Google Scholar]
- Mo Y., Zhang X., Yao Y., Duan C., Ye L., Shi Z., Zhao Z., Li X. Construction of chiral isotetronic acid-fused thiochromane via doubly annulative strategy. J. Org. Chem. 2021;86:4448–4456. doi: 10.1021/acs.joc.0c02878. [DOI] [PubMed] [Google Scholar]
- Modrocka V., Veverkova E., Meciarova M., Sebesta R. Bifunctional amine-squaramides as organocatalysts in michael/hemiketalization reactions of β,γ-unsaturated α-ketoesters and α,β-unsaturated ketones with 4-hydroxycoumarins. J. Org. Chem. 2018;83:13111–13120. doi: 10.1021/acs.joc.8b01847. [DOI] [PubMed] [Google Scholar]
- Muthusamy S., Kesavan V. Asymmetric cycloaddition reactions of oxindole α-keto esters via cascade dienamine-enamine and trienamine strategies. E. J. Org. Chem. 2019;2019:4046–4055. doi: 10.1002/ejoc.201900567. [DOI] [Google Scholar]
- Muthusamy S., Prakash M., Ramakrishnan C., Gromiha M.M., Kesavan V. Organocatalytic enantioselective assembly of spirooxindole-naphthopyrans through tandem friedel-crafts type/hemiketalization. ChemCatChem. 2016;8:1708–1712. doi: 10.1002/cctc.201600087. [DOI] [Google Scholar]
- Obregón-Zúñiga A., Guerrero-Robles M., Juaristi E. Chiral imidazolium ionic liquids derived from (s)-prolinamine as organocatalysts in the asymmetric Michael reaction and michael-aldol cascade reaction under solvent-free conditions. E. J. Org. Chem. 2017;2017:2692–2697. doi: 10.1002/ejoc.201700133. [DOI] [Google Scholar]
- Peňaška T., Palchykov V., Rakovský E., Addová G., Šebesta R. Stereoselective organocatalytic construction of spiro oxindole pyrrolidines using unsaturated α-ketoesters and α-ketoamides. E. J. Org. Chem. 2021;2021:1693–1703. doi: 10.1002/ejoc.202100022. [DOI] [Google Scholar]
- Pratap Reddy Gajulapalli V., Lokesh K., Vishwanath M., Kesavan V. Organocatalytic construction of spirooxindole naphthoquinones through Michael/hemiketalization usingl-proline derived bifunctional thiourea. RSC Adv. 2016;6:12180–12184. doi: 10.1039/c5ra25025k. [DOI] [Google Scholar]
- Qin J., Zhang Y., Liu C., Zhou J., Zhan R., Chen W., Huang H. Asymmetric inverse-electron-demand diels-alder reaction of β,γ-unsaturated amides through dienolate catalysis. Org. Lett. 2019;21:7337–7341. doi: 10.1021/acs.orglett.9b02629. [DOI] [PubMed] [Google Scholar]
- Ren H., Song X.Y., Wang S.R., Wang L., Tang Y. Highly enantioselective nickel-catalyzed oxa-[3 + 3]-annulation of phenols with benzylidene pyruvates for chiral chromans. Org. Lett. 2018;20:3858–3861. doi: 10.1021/acs.orglett.8b01442. [DOI] [PubMed] [Google Scholar]
- Rong C., Pan H., Liu M., Tian H., Shi Y. A Facile approach to optically active hydroquinoline-2-carboxylates by a one-pot asymmetric michael/transamination/cyclization process. Chem. Eur. J. 2016;22:2887–2891. doi: 10.1002/chem.201503327. [DOI] [PubMed] [Google Scholar]
- Sinha D., Biswas A., Singh V.K. Chiral phosphine-silver(i) complex catalyzed enantioselective interrupted Feist-Benary reaction with ynones: the aldol-cycloisomerization cascade. Org. Lett. 2015;17:3302–3305. doi: 10.1021/acs.orglett.5b01468. [DOI] [PubMed] [Google Scholar]
- Sun B.-B., Chen J.-B., Zhang J.-Q., Yang X.-P., Lv H.-P., Wang Z., Wang X.-W. Organo-catalyzed asymmetric cascade annulation reaction for the construction of bi-spirocyclic pyrazolone and oxindole derivatives. Org. Chem. Front. 2020;7:796–809. doi: 10.1039/d0qo00001a. [DOI] [Google Scholar]
- Sun J., Hu Y., Li Y., Zhang S., Zha Z., Wang Z. Copper-catalyzed chemoselective and enantioselective friedel-crafts 1,2-addition of pyrrole with β,γ-unsaturated α-ketoesters. J. Org. Chem. 2017;82:5102–5110. doi: 10.1021/acs.joc.7b00159. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wang Z., Wu S., Zhang Y., Shi F. Lewis acid-catalyzed [4 + 2] cycloaddition of 3-alkyl-2-vinylindoles with β,γ-unsaturated α-ketoesters. Green. Synth. Catal. 2022 doi: 10.1016/j.gresc.2021.11.003. [DOI] [Google Scholar]
- Tang Q., Fu K., Ruan P., Dong S., Su Z., Liu X., Feng X. Asymmetric catalytic formal 1,4-allylation of β,γ-unsaturated α-ketoesters: allylboration/oxy-cope rearrangement. Angew. Chem. Int. .Ed. 2019;58:11846–11851. doi: 10.1002/anie.201905533. [DOI] [PubMed] [Google Scholar]
- Taylor J.E., Davies A.T., Douglas J.J., Churchill G., Smith A.D. Enantioselective NHC-catalysed redox [4 + 2]-hetero-Diels-Alder reactions using α-aroyloxyaldehydes and unsaturated ketoesters. Tetrahedron. 2017;28:355–366. doi: 10.1016/j.tetasy.2017.01.002. [DOI] [Google Scholar]
- Thirupathi N., Wei F., Tung C.H., Xu Z. Divergent synthesis of chiral cyclic azides via asymmetric cycloaddition reactions of vinyl azides. Nat. Commun. 2019;10:3158. doi: 10.1038/s41467-019-11134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B.M., Hung C.J., Mata G. Dinuclear metal-prophenol catalysts: development and synthetic applications. Angew. Chem. Int. .Ed. 2020;59:4240–4261. doi: 10.1002/anie.201909692. [DOI] [PubMed] [Google Scholar]
- Tukhvatshin R.S., Kucherenko A.S., Nelyubina Y.V., Zlotin S.G. Conjugate addition of carbon acids to β,γ-unsaturated α-keto esters: product tautomerism and applications for asymmetric synthesis of benzo[a]phenazin-5-ol derivatives. J. Org. Chem. 2019;84:13824–13831. doi: 10.1021/acs.joc.9b02021. [DOI] [PubMed] [Google Scholar]
- Vishwanath M., Vinayagam P., Gajulapalli V.P.R., Kesavan V. Asymmetric organocatalytic assembly of oxindoles fused with spiro-3,4-dihydropyrans with three contiguous stereocenters consisting of vicinal quaternary centers. Asian J. Org. Chem. 2016;5:613–616. doi: 10.1002/ajoc.201600051. [DOI] [Google Scholar]
- Wang L., Lv J., Zhang L., Luo S. Catalytic Regio- and enantioselective [4+2] annulation reactions of non-activated allenes by a chiral cationic indium complex. Angew. Chem. Int. .Ed. 2017;56:10867–10871. doi: 10.1002/anie.201704020. [DOI] [PubMed] [Google Scholar]
- Wei A.J., Nie J., Zheng Y., Ma J.A. Ni-catalyzed highly chemo-, regio-, and enantioselective decarboxylative aldol reaction of β,γ-unsaturated α-ketoesters with β-ketoacids. J. Org. Chem. 2015;80:3766–3776. doi: 10.1021/jo502741z. [DOI] [PubMed] [Google Scholar]
- Xiao X., Shao B.X., Lu Y.J., Cao Q.Q., Xia C.N., Chen F.E. Recent advances in asymmetric organomulticatalysis. Adv. Synth. Catal. 2020;363:352–387. doi: 10.1002/adsc.202000961. [DOI] [Google Scholar]
- Xie L., Yu X., Li J., Zhang Z., Qin Z., Fu B. Nickel(II)-catalyzed enantioselective 1,3-dipolar cycloaddition of nitrones with α,β-unsaturated acylcarboxylates. E. J. Org. Chem. 2017;2017:657–661. doi: 10.1002/ejoc.201601540. [DOI] [Google Scholar]
- Xiong Q., Lin L., Zhao X., Lang J., Liu X., Feng X. Zinc(II)-catalyzed asymmetric diels-alder reaction of (E)-1-phenyl dienes with β,γ-unsaturated α-ketoesters. J. Org. Chem. 2018;83:12527–12534. doi: 10.1021/acs.joc.8b01771. [DOI] [PubMed] [Google Scholar]
- Xu C., Wang K., Li D., Lin L., Feng X. Asymmetric synthesis of oxa-bridged oxazocines through a catalytic rh(ii)/zn(ii) relay [4 + 3] cycloaddition reaction. Angew. Chem. Int. .Ed. 2019;58:18438–18442. doi: 10.1002/anie.201910898. [DOI] [PubMed] [Google Scholar]
- Xu J., Hu L., Hu H., Ge S., Liu X., Feng X. Enantioselective vinylogous Michael-Aldol reaction to synthesize spirocyclohexene pyrazolones in aqueous media. Org. Lett. 2019;21:1632–1636. doi: 10.1021/acs.orglett.9b00168. [DOI] [PubMed] [Google Scholar]
- Xu Y., Yang Z., Ding B., Liu D., Liu Y., Sugiya M., Imamoto T., Zhang W. Asymmetric Michael addition of diphenylphosphine to β,γ-unsaturated α-keto esters catalyzed by a P-stereogenic pincer-Pd complex. Tetrahedron. 2015;71:6832–6839. doi: 10.1016/j.tet.2015.07.026. [DOI] [Google Scholar]
- Yang K., Ma Z., Tong H.-X., Sun X.-Q., Hu X.-Y., Li Z.-Y. Asymmetric Michael addition reactions catalyzed by a novel upper-rim functionalized calix[4]squaramide organocatalyst. Chin. Chem. Lett. 2020;31:3259–3262. doi: 10.1016/j.cclet.2020.02.057. [DOI] [Google Scholar]
- Yao Q., Yu H., Zhang H., Dong S., Chang F., Lin L., Liu X., Feng X. Lewis acid catalyzed asymmetric [4 + 2] cycloaddition of cyclobutenones to synthesize α,β-unsaturated delta-lactones. Chem. Commun. 2018;54:3375–3378. doi: 10.1039/C8CC01040D. [DOI] [PubMed] [Google Scholar]
- Yao W., Dou X., Lu Y. Highly enantioselective synthesis of 3,4-dihydropyrans through a phosphine-catalyzed [4 + 2] annulation of allenones and β,γ-unsaturated α-keto esters. J. Am. Chem. Soc. 2015;137:54–57. doi: 10.1021/ja5109358. [DOI] [PubMed] [Google Scholar]
- Yin S.-J., Zhang S.-Y., Zhang J.-Q., Sun B.-B., Fan W.-T., Wu B., Wang X.-W. Organocatalytic tandem enantioselective Michael-cyclization of isatin-derived β,γ-unsaturated α-ketoesters with 3-hydroxy-4H-chromen-4-one or 2-hydroxy-1,4-naphthoquinone derivatives. RSC Adv. 2016;6:84248–84254. doi: 10.1039/c6ra17400k. [DOI] [Google Scholar]
- Zhang J.-Q., Li N.-K., Yin S.-J., Sun B.-B., Fan W.-T., Wang X.-W. Chiral N-heterocyclic carbene-catalyzed asymmetric michael-intramolecular aldol-lactonization cascade for enantioselective construction of β-propiolactone-fused spiro[cyclopentane-oxindoles] Adv. Synth. Catal. 2017;359:1541–1551. doi: 10.1002/adsc.201601259. [DOI] [Google Scholar]
- Zhang M.L., Wu Z.J., Zhao J.Q., Luo Y., Xu X.Y., Zhang X.M., Yuan W.C. Michael addition-lactonization of arylacetyl phosphonate to β,γ-unsaturated α-keto esters for the synthesis of chiral syn-3,4-dihydropyranones and 5,6-dihydropyranones. Org. Lett. 2016;18:5110–5113. doi: 10.1021/acs.orglett.6b02558. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lv J., Luo S. Enantioselective Diels-Alder reaction of anthracene by chiral tritylium catalysis. Beilstein J. Org. Chem. 2019;15:1304–1312. doi: 10.3762/bjoc.15.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Greenhalgh M.D., Slawin A.M.Z., Smith A.D. Tandem sequential catalytic enantioselective synthesis of highly-functionalised tetrahydroindolizine derivatives. Chem. Sci. 2020;11:3885–3892. doi: 10.1039/d0sc00432d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu X.-Y., Han J. Chiral iminophosphorane catalyzed asymmetric Henry reaction of α,β-alkynyl ketoesters. Chin. Chem. Lett. 2019;30:1519–1522. doi: 10.1016/j.cclet.2019.04.042. [DOI] [Google Scholar]
- Zhang Y., Yang N., Liu X., Guo J., Zhang X., Lin L., Hu C., Feng X. Reversal of enantioselective friedel-crafts C3-alkylation of pyrrole by slightly tuning the amide units of N,N'-dioxide ligands. Chem. Commun. 2015;51:8432–8435. doi: 10.1039/c4cc10055g. [DOI] [PubMed] [Google Scholar]
- Zhang Z.-G., Fu J.-H., Sha F., Wu X.-Y. Highly chemo- and enantioselective vinylogous aldol/cyclization cascade reaction to construct chiral 5,6-dihydropyran-2-ones. Tetrahedron. 2018;74:3557–3563. doi: 10.1016/j.tet.2018.05.011. [DOI] [Google Scholar]
- Zhao L., Yao E.Z., Chai G.L., Ma S.Y., Chang J. Organocatalyzed enantioselective conjugate addition of boronic acids to β,γ-unsaturated α-ketoesters. J. Org. Chem. 2021;86:18211–18223. doi: 10.1021/acs.joc.1c02497. [DOI] [PubMed] [Google Scholar]
- Zhao X., Mei H., Xiong Q., Fu K., Lin L., Liu X., Feng X. Highly enantioselective construction of carbazole derivatives via [4 + 2] cycloaddition of silyloxyvinylindoles and β,γ-unsaturated α-ketoesters. Chem. Commun. 2016;52:10692–10695. doi: 10.1039/c6cc05328a. [DOI] [PubMed] [Google Scholar]
- Zhi Y., Huang J., Liu N., Lu T., Dou X. Rhodium-catalyzed asymmetric conjugate alkynylation of β,γ-unsaturated α-ketoesters. Org. Lett. 2017;19:2378–2381. doi: 10.1021/acs.orglett.7b00909. [DOI] [PubMed] [Google Scholar]
- Zhong X., Lv J., Luo S. Enantio-and diastereoselective cyclopropanation of β,γ-unsaturated α-ketoester by a chiral phosphate/indium(iii) complex. Org. Lett. 2017;19:3331–3334. doi: 10.1021/acs.orglett.7b01577. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liu X.-L., Zhou H.-J., Zhou W., Tian Y.-P., Wang J.-X. Catalytic asymmetric domino michael/annulation reaction of bifunctional chromone synthons with β,γ-unsaturated α-keto esters: rapid access to polysubstituted spirocyclic hexahydroxanthones. Synthesis. 2020;52:3047–3057. doi: 10.1055/s-0040-1707340. [DOI] [Google Scholar]
- Zhu X.-Y., Chen J.-R., Lu L.-Q., Xiao W.-J. An efficient synthesis of enol phosphates via organic base-promoted addition of phosphites to 4-oxo-enoates. Tetrahedron. 2012;68:6032–6037. doi: 10.1016/j.tet.2012.05.021. [DOI] [Google Scholar]