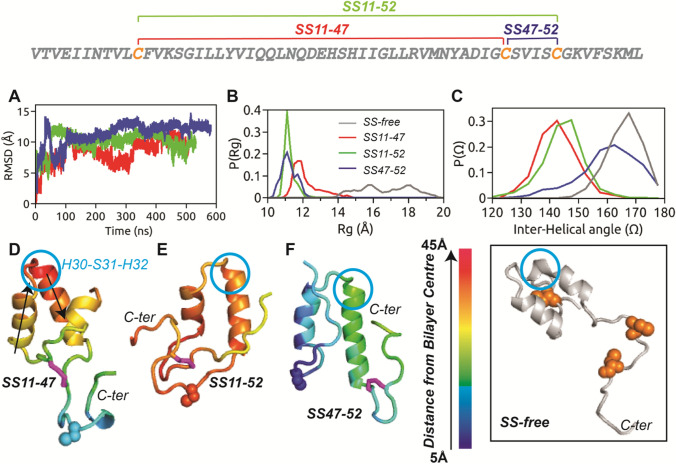
Fig. 1.
The primary sequence of HAV-2B peptide is shown with the three cysteine residues highlighted in orange, which can form the three possible SS-bonded states. a Cα-RMSD of HAV-2B peptide in different SS-bonded states with respect to corresponding initial configurations, showing flat trajectory over the last 200 ns. The equilibrium distributions of b radius of gyration, and c inter-helical angle, of disulphide-bonded states of HAV-2B peptide: SS11-47 (red), SS11-52 (green) and SS47-52 (blue). and of SS-free HAV-2B peptide (grey) are shown for comparison. The final snapshots of d SS11-47, e SS11-52 and f SS47-52 peptides are illustrated with colour codes representing insertion depth into POPC bilayer; the smaller the value, the deeper is the insertion. The SS-bond is shown in stick representation (magenta), whilst the cysteine in reduced thiol state is shown in spheres. The arrows in (d) indicate the vectors corresponding to helical axes, used for calculating the inter-helical angle, . The residue triad, H30-S31-H32 which undergoes conformational transition upon inclusion of disulphide bond is indicated by blue circle. The inset shows the largely extended conformation of SS-free HAV-2B peptide and the position of cysteine residues (spheres in orange)