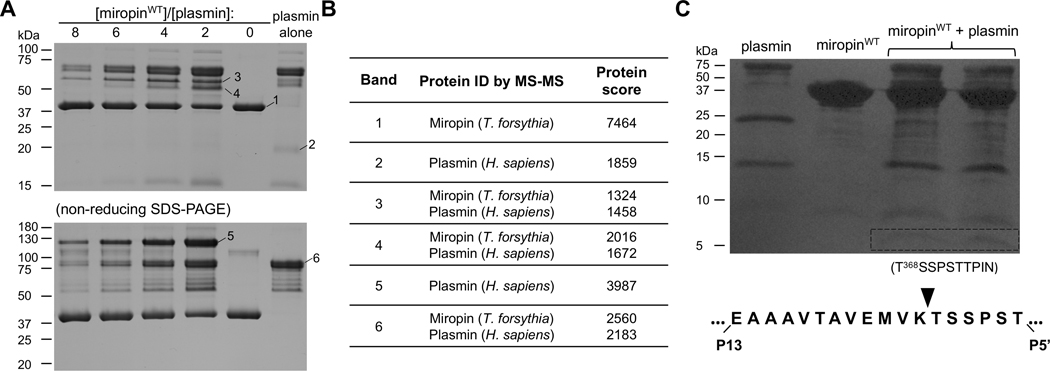
Figure 3:
Miropin forms stable covalent complexes after cleavage of the Lys368(P2)-Ala(P1) reactive site 675 peptide bond. A) The protease and inhibitor were incubated together at different molar ratios or alone, and then separated by SDS-PAGE under reducing (upper gel) and non-reducing (lower gel) conditions. Selected bands were excised and subjected to mass spectrometry analysis to identify the proteins in the bands (Table 1). B) Miropin was incubated with plasmin until the reaction was stopped via addition of reducing sample buffer. The proteins were then resolved via SDS-PAGE and electrotransferred onto a PVDF membrane. The stained protein bands with a molecular weight of ~5 kDa (framed) were excised and subjected to N-terminal sequence analysis, which allowed identification of the RBS, K368 (P2)-Ala (P1), for plasmin within the miropin reactive center loop.