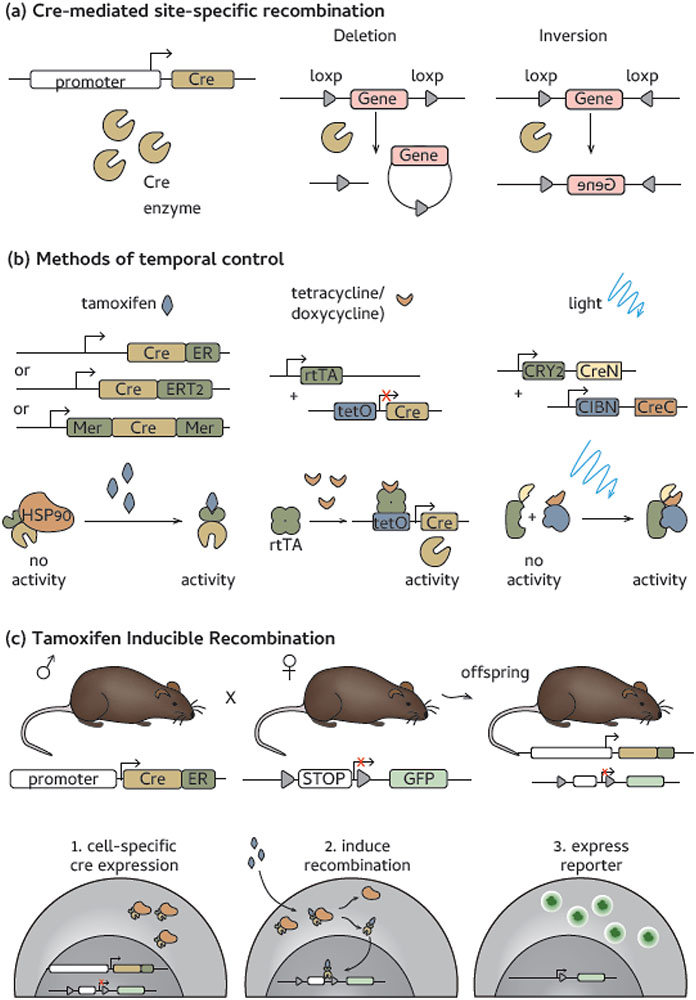
Figure 1. How fate-mapping mice are made.
(a) Cre cassettes can be placed under the control of cell specific promoters. Cre is a site-specific recombinase that recognizes loxP sites as targets for DNA cleavage. Recognition of loxP sites in the same direction result in gene cassette deletion, while gene inversion occurs when loxP sites are placed in opposing directions. (b) Temporal expression is key for most current fate-mapping models. Tamoxifen mediated induction is a common method of inductions and is accomplished through one of three drivers: Cre-estrogen receptor (ER), Cre-ERT2 [10] or MerCreMer [11]. Other methods of temporal control include tetracycline induction [124] or split-Cre constructs that can be activated by a protein conformational change induced by light exposure [118]. (c) A fully functional fate-mapping model is often made by crossing driver and reporter lines to make a F1 generation with each transgene. In offspring, (1) Cre expression is cell specific, upon addition of tamoxifen, (2) the Cre-ER fusion protein translocates to the nucleus and mediates the recombination event and this (3) allows the reporter protein(s) to be expressed in a cell specific manner [10].