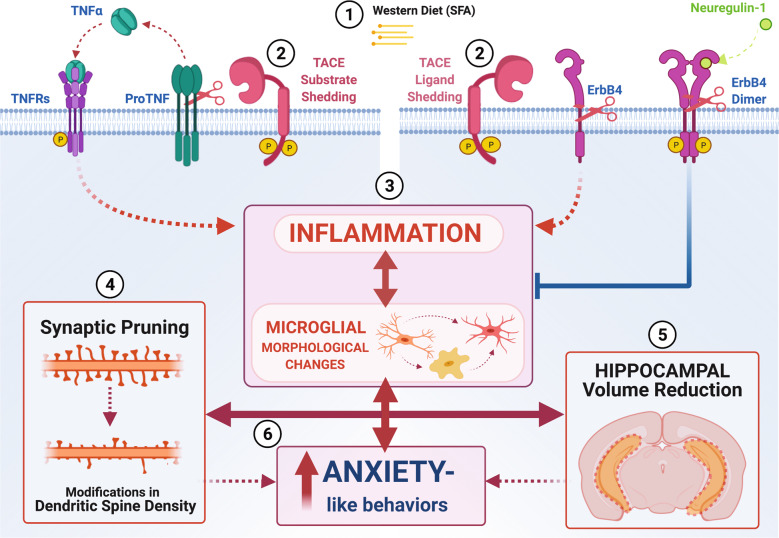
Fig. 8. Working model unifying neurodevelopmental and neuroinflammatory hypotheses implicated in obesity-mediated hippocampal dysfunction.
1 It is plausible that exposure to obesogenic diets rich in saturated fatty acids (SFAs) increase TACE/ADAM17 sheddase expression and activities, (2) leading to dysregulation of neuroinflammatory (e.g., tumor necrosis factor alfa, TNF-α) and neurodevelopmental (ErbB4) mediators. (3) TNF receptor (TNFR) activation and prolonged ErbB4 cleavage tip the inflammatory balance to a pro-inflammatory state associated with microglial activation. (4) Neuroinflammation and microglial activation promote synaptic pruning and remodeling. (5) Disruption in microglia-synapse interactions contributes to synapse loss, dysfunction, and, consequently, hippocampal volume atrophy. (6) In summary, evidence in this study supports the conclusion that microglia respond to trophic factors and secrete cytokines, which are critical for normative synaptic plasticity in the maturing brain [137, 138]. Our data suggest that an imbalance in the levels of cytokines and neurotrophic factors during early pre-obesity states can impair microglia, synaptic plasticity, which may result in anxiety. Although our analyses focused on microglia and provided limited directionality of the effects, this dataset provides a rich resource for identifying meaningful synaptic targets in response to an obesogenic WD.