Figure 1.
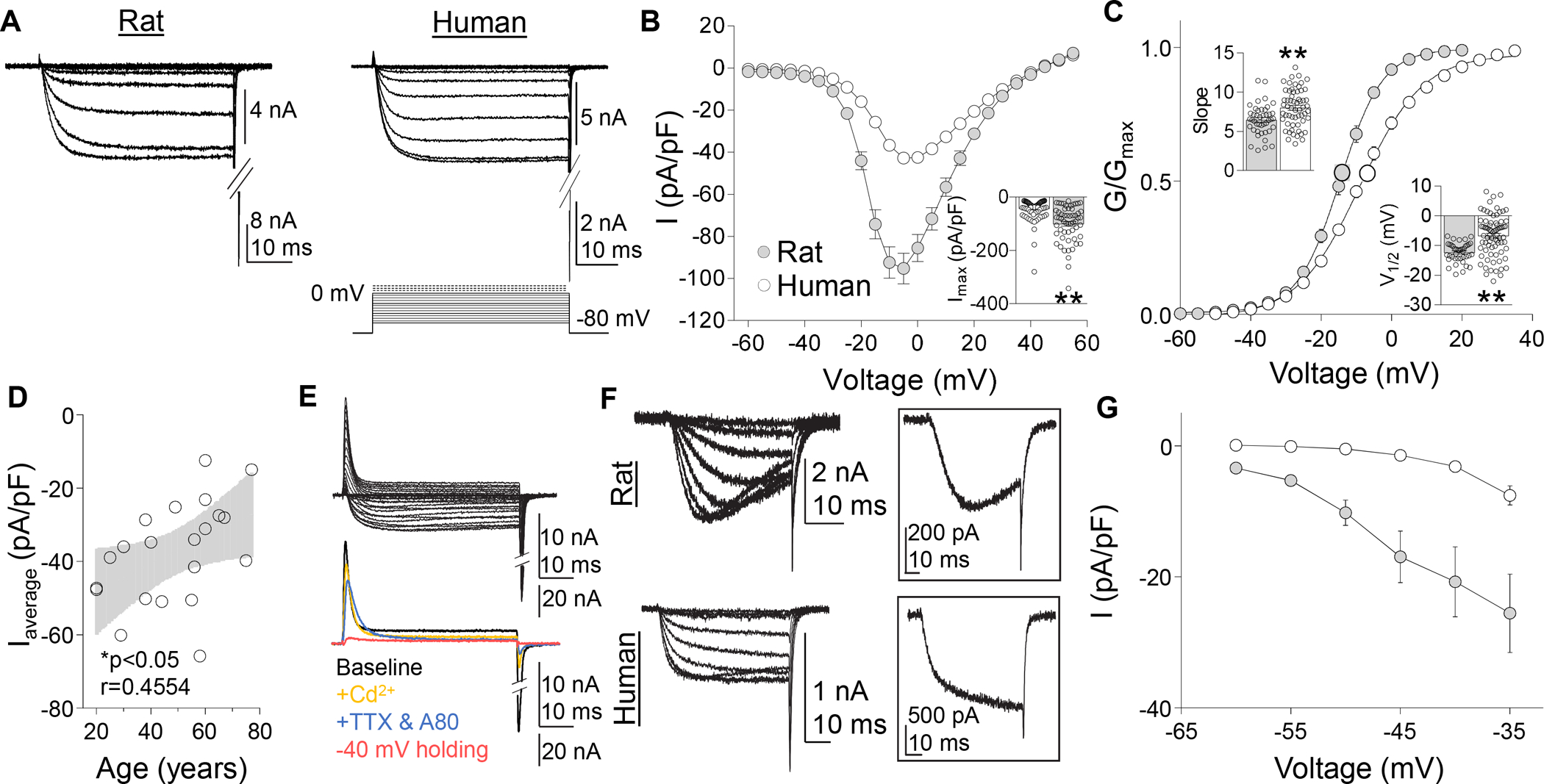
Voltage gated Ca2+ currents in rat and human DRG neurons. (A) A series of depolarizing voltage steps were used to evoke HVA currents were evoked in rat and human DRG neurons. (B) Pooled HVA I-V data from human and rat DRG neurons. Inset: Variability in peak inward HVA current evoked in human and rat DRG neurons (n = 87 human, 57 rat), where each point represents a single neuron. (C) Pooled G-V data from the same neurons plotted in (B). G-V data for each neuron was used to estimate Gmax, the voltage-dependence of activation (slope), and the voltage (V1/2) at which conductance was half of Gmax. Insets: Variability in slope and V1/2. (D) There was a negative correlation between the mean peak current density (Imax pA/pF) per donor (n = 22) and donor age. Black line is a linear fit of the data with grey area the 95% confidence interval. (E) With time in culture, there was an increase in a voltage-dependent transient outward current (top traces), that was not Ca2+ dependent, but was abolished by blockade of TTX-sensitive and -insensitive voltage gated sodium currents with 300 nM TTX, 300 nM A803467, and changing the holding potential is −40 mV (bottom traces). (F) LVA currents (activation threshold < −35 mV) were detected in a subpopulation of both human and rat DRG neurons. LVA currents detected in rat DRG neurons were slowly activating, slowing inactivating, and slowly deactivating. No open-state inactivation was detected in human LVA currents. Inset: LVA current evoked at −35 mV in rat (top trace) and human (bottom trace) DRG neurons. (G) Pooled I-V data for LVA currents detected in human (n = 18) and rat (n = 18) DRG neurons. Currents were evoked following a 500 ms pre-pulse to −100 mV. *p < 0.05, **p < 0.01. Data are mean ± SEM.
