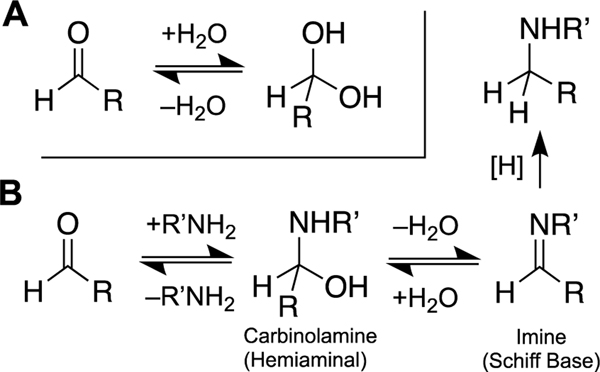
Scheme 2.
Reversible addition of nucleophiles to aldehydes. Panel A: Reversible hydration of an aldehyde. Panel B: The reaction of an amine with an aldehyde reversibly generates an imine. When the reaction is conducted in the presence of a water-compatible hydride donor such as NaBH3CN, the equilibria are drawn irreversibly forward to generate good yields of the corresponding alkylamine. This type of reaction is known as a “reductive amination of an aldehyde”.