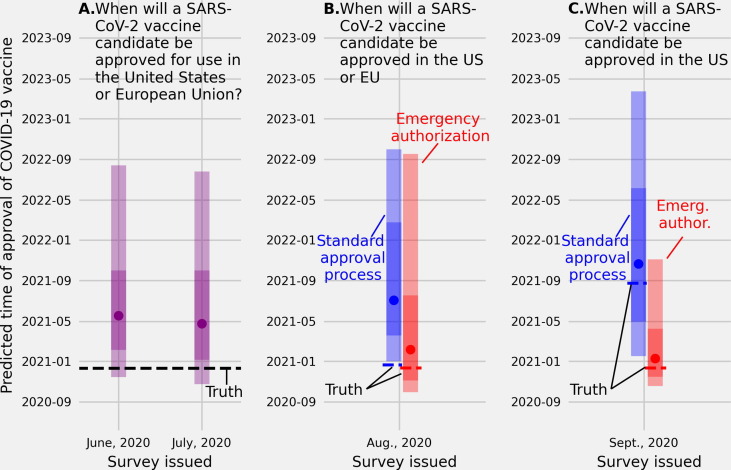
Fig. 3.
(A.) Linear pool predictive percentiles made in June and in July, 2020 for the date when SARS-CoV-2 vaccine will be approved for use in the US or European Union (EU). (B.) Linear pool predictive percentiles for the date a SARS-CoV-2 vaccine will be approved for use in the US or EU through a standard approval process (blue) or an emergency use authorization (red), and (C.) linear pool predictive percentiles for the date a SARS-CoV-2 vaccine will be approved for use specifically in the US through a standard approval process (blue) or an emergency use authorization (red). The linear pool median predictions made in June and July for when a SARS-CoV-2 candidate would be approved in the US or EU were many months later than the truth (May, 2020 and April, 2020 vs Dec., 2020). Linear pool median predictions of the date of emergency and standard approval of a SARS-CoV-2 vaccine in the US or EU were less accurate than predictions of approval dates for the US only. Environmental cues, time between when the forecast was made and the truth, or how the question was asked, may have impacted predictive accuracy.