Summary
Background
The infantile spasms syndrome is an early-onset epileptic encephalopathy presenting in the first 2 years of life, often with severe developmental consequences. The role of the gut microbiota and metabolism in infantile spasms remains unexplored.
Methods
Employing a brain injury neonatal rat model of infantile spasms intractable to anticonvulsant medication treatments, we determined how the ketogenic diet and antibiotics affected specific microbial communities and the resultant circulating factors that confer spasms protection in the infantile spasms model. To confirm a role of kynurenine metabolism pathway in spasms protection, indoleamine 2,3-dioxygenase 1 was pharmacologically inhibited and comprehensive metabolomics was applied.
Findings
We show that antibiotics reduced spasms and improved the effectiveness of the ketogenic diet when given in combination. Examination of the gut microbiota and metabolomics showed the downregulation of indoleamine 2,3-dioxygenase 1 and upregulation of hippocampal kynurenic acid, a metabolite with antiepileptic effects. To further test the involvement of indoleamine 2,3-dioxygenase 1, a specific antagonist 1-methyltryptophan and minocycline, an antibiotic and inhibitor of kynurenine formation from tryptophan, were administered, respectively. Both treatments were effective in reducing spasms and elevating hippocampal kynurenic acid. A fecal microbiota transplant experiment was then performed to examine the contribution of the gut microbiota on spasm mitigation. Transplant of feces of ketogenic diet animals into normal diet animals was effective in reducing spasms.
Interpretation
These results highlight the importance of tryptophan-kynurenine metabolism in infantile spasms and provide evidence for new-targeted therapies such as indoleamine 2,3-dioxygenase 1 inhibition or microbiota manipulation to promote kynurenic acid production as a strategy to reduce spasms in infantile spasms.
Funding
This study was funded by the Alberta Children's Hospital Research Institute and the Owerko Centre.
Keywords: Infantile spasms; Gut microbiota; Ketogenic diet; Indoleamine 2,3-dioxygenase 1
Research in context.
Evidence before this study
Infantile spasms (IS) is a debilitating epileptic encephalopathy occurring in infancy. Patients with IS are at high risk of mortality and severe long-term neurodevelopmental disability if effective treatments are not instituted early. However currently available treatments (ACTH and vigabatrin) are often ineffective and are associated with toxic side effects. A growing body of evidence shows the essential role of the gut microbiome in regulating diverse physiological processes including neural function via the gut-brain axis. In an infant model of symptomatic IS induced in neonatal rats we've previously reported that the ketogenic diet is efficient in reducing spasms occurrence. However, the role of gut microbiota and metabolism remains unclear.
Added value of this study
Employing a brain injury model of IS intractable to currently available firstline treatments, we show that antibiotics alone reduced seizures and improved the effectiveness of the ketogenic diet (KD) when given in combination. When examining the metabolomics and microbiome profiles of the animals (antibiotics, KD, KD + antibiotics), our results revealed alterations to the kynurenine pathway including upregulation of kynurenic acid and a downregulation of indoleamine 2,3-dioxygenase 1 (IDO1) activity as revealed by a reduction in the kynurenine /tryptophan ratio. Inhibition of IDO1 with a specific antagonist and minocycline (single antibiotic) worked to elevate kynurenic acid concentrations and reduce seizures.
Implications of all the available evidence
These data provide evidence for new-targeted therapies such as IDO1 inhibitor or microbiota manipulation against infantile spasms.
Alt-text: Unlabelled box
Introduction
Infantile spasms (IS) is a debilitating epileptic encephalopathy that affects 1/5000 children.1 Principal clinical features include frequent epileptic spasms occurring in infancy, abnormal inter-ictal EEG termed hypsarrhythmia and developmental arrest or regression.1 Patients with IS are at high risk of mortality and severe long-term neurodevelopmental disability if effective treatments are not instituted early.2 However currently available treatments (ACTH and vigabatrin) are often ineffective and are associated with toxic side effects.3
Patients with IS have abnormal tryptophan (TRP) levels that are normalized in those who respond to ACTH.4 Subsequent studies have reported significantly lower levels of kynurenine (KYN) and 5-hydroxyindoleacetic acid in the cerebrospinal fluid of patients with IS.5, 6, 7 These studies support a role for abnormal TRP metabolism in the pathogenesis of IS. TRP is an essential amino acid that is the sole precursor of serotonin and the neuroendocrine hormone melatonin. TRP is metabolized by two main pathways; either conversion to serotonin (5-HT; 1% of metabolism) or metabolism via the KYN pathway (95% of metabolism) by indoleamine 2,3-dioxygenase 1 (IDO1).8 The metabolic products of KYN are quinolinic acid (QA) or kynurenic acid (KA), which subsequently activates or inhibits neurotransmission, respectively.8 Notably, QA is lastly converted to NAD+, which regulates important metabolic enzymes.
Pathological imbalance in the production of QA versus KA can have profound effects on cerebral excitability.9 Intraventricular administration of KYN is well documented to potentiate seizures,10,11 while administration of the ketogenic diet (KD), which is an alternative treatment for patients with IS12, 13, 14 reduces KYN in both plasma as well as the KYN/TRP ratio in the hippocampus.15 Inflammation is a known inducer of the KYN pathway through IDO1,16 the rate-limiting enzyme in the KYN pathway in the brain.
The role of the gut microbiota in mediating TRP availability and metabolism is increasingly recognized.17 More than 90% of the body's 5-HT is synthesized in the gut and studies show that both antibiotic administration and germ-free mice have severely reduced levels of 5-HT in both the serum and colon compared to conventionally-colonized controls.18 The specific mechanisms whereby the gut microbiota communicates with the brain to modulate spasms remain elusive. While gut dysbiosis has been reported in patients with drug-resistant epilepsy,19 analysis of fecal microbiota in children with refractory epilepsy are inconsistent, likely due to varied etiologies and treatment regimes.20, 21, 22
Traditionally, IS are classified into symptomatic, cryptogenic and idiopathic. To date, there is a paucity of basic knowledge about the role of the gut microbiota in the context of symptomatic IS. Herein, we employ the triple-hit model,23 to examine the emerging link between the gut microbiota and TRP metabolism in IS. We sought to determine how the KD and antibiotics affected specific microbial communities and the resultant circulating factors that confer spasms protection in the IS model. Comprehensive metabolomics profiling revealed alterations to the KYN pathway including upregulation of KA and a downregulation of IDO1 activity as revealed by a reduction in the KYN/TRP ratio. To confirm a role of this pathway in spasms protection, IDO1 was pharmacologically inhibited with positive results. Taken together, data show that targeting the microbiota and its downstream targets involving TRP metabolism may serve as an approach to modulating spasms occurrence in IS.
Methods
Animals
All procedures were performed under the guidelines of the Canadian Council on Animal Care and had ethics approval by the Health Sciences Animal Care Committee at the University of Calgary (Approval ID. AC16-0271). All animal experiments were conducted on offspring of Sprague-Dawley rats. The neonatal Sprague-Dawley rats were obtained from Sprague Dawley females (Charles River Laboratories) bred in house, in full compliance with the institutional guidelines of the University of Calgary's Health Sciences Animal Care Committee. The day of birth was considered as P0 and on P3 the litters were culled to 8 pups. Pups were divided into two experimental settings: (1) lesion pups that received doxorubicin/lipopolysaccharide/p-chlorophenylalanine, (2) lesion pups that received saline. The triple-hit model is the only fully validated model of symptomatic infantile spasms that mimics IS in infants.24 On P4, intracerebral infusions of doxorubicin and lipopolysaccharide were made stereotaxically under hypothermia anesthesia. Pups were positioned in a stereotaxic frame for neonatal rat surgery (Benchmark Angle One, MyNeurolab.com, St. Louis MO). Doxorubicin was injected into the right lateral ventricle followed by lipopolysaccharide into the right parietal cortex. The following coordinates were used: doxorubicin (5 µg/2.5 µL): 2.68 mm anterior to lambda; 1.1 mm lateral to sagittal suture; 3.3 mm deep; lipopolysaccharide (3 µg/1.5 µL): 2.55 mm anterior to lambda; 1 mm lateral to sagittal suture; 1.7 mm deep. After the injections the skin was closed with Vetbond© and the pups were allowed to recover on a heating pad before giving access to food and water. At P5 morning, pups were injected with p-chlorophenylalanine 200 mg/kg i.p. Sterile saline was used as the vehicle for the three injections. Pups were individually placed in beakers warmed in a water bath (45 °C) and filled with bedding material (31–33 °C) in a specific pathogen free facility. Artificial rearing was started once the pups have fully recovered from the anesthesia. Pups were randomly assigned (based on body weight) to either a normal milk diet (1.7:1, fats to carbohydrate/protein ratio) or the ketogenic diet (4:1, fats to carbohydrate/protein ratio; made in house) from P4 to P7 or P12. Loss of body weight < 20%, abdominal distension, and/or an infection at the incision site that prevents us from suturing were assumed a humane endpoint. Rats meeting the humane endpoints were excluded from the study. Treatment and measurements were performed at the same time of experimental endpoints and in a random order to minimize potential confounders.
Antibiotic treatment
On P5, the pups were randomly assigned for antibiotic-treated or untreated group. A broad-spectrum antibiotic mixture was added in the milk at the daily dosage of 20 mg/kg vancomycin (V2002, Sigma), 50 mg/kg neomycin (N6386, Sigma), 50 mg/kg ampicillin (A9393, Sigma) and 10 mg/kg metronidazole (M3761, Sigma). All dosages refer to the available therapeutic dosages used in clinics. Body weight was measured twice per day.
1-Methyltryptophan (1-MT) administration
1-MT was administered subcutaneously at the dose of 50 mg/kg as previously described.25 The injections were given twice daily twice daily at 12 h intervals from P5 to P6 and once at P7 morning. 1-MT was dissolved in 0.1 M NaOH and adjusted pH to using 1 M HCl.
Minocycline treatment
On P5, the pups were randomly assigned to groups treated with or without minocycline. According to previous reports,26,27 a dosage at 50 mg/kg body weight was chosen for administration. Minocycline was given orally with milk.
Fecal microbiota transplantation
Fresh feces were collected from P7 rats fed with ketogenic diet. 200 µL 20% glycerol was added to 0.02 g feces and thoroughly re-suspended to prepare fecal supernatants. Each mixture was further centrifuged at 376 xg for 5 min at 4 °C to remove sediment of undigested dietary materials. 100 µL of the supernatant was orally gavaged to recipient rats twice on P4 after surgery and at P5 in the morning.
Behavioral spasms
The behaviors of the pups were continuously monitored from P4 (surgery day) to P7 (day of sacrifice). The behavioral spasms characterized by rapid extension and flexion movements were recorded using video system with Sirenia software (Pinnacle Technology, Lawrence, KS, USA), based on previously described criteria.24 A spasm was blindly recorded when there is an abrupt onset of flexion of the trunk with forward tonic extension of the limbs at the height of the spasm (flexion spasms), or a sudden hyper-extension of the back with tonic posturing of the limbs (extension spasms).
Bacterial genomic DNA extraction and 16S rRNA high-throughput sequencing
The bacterial genomic DNAs from fecal samples of P7 and 12 rats were isolated using the FastDNA® SPIN Kit for Feces (Cat. No. 116570200; MP Biomedicals, Santa Ana, CA) and quantified using the high-sensitivity dsDNA Qubit Kit (Invitrogen, Carlsbad, CA, USA). The 16S V3 and V4 region was amplified with 12.5 ng extracted DNA, 5 μL barcoded fusion primers,28 12.5 μL 2X KAPA HiFi Hot Start Ready Mix (KAPA Biosystems). The DNA libraries were prepared using the Nextera XT DNA Sample Preparation (Illumina) and the amplicon was purified using AMPure XP beads. Sequencing was performed on an Illumina MiSeq platform with the MiSeq V3 600 cycle sequencing kit for paired-end sequencing. All the procedues are conducted in the Core DNA facility of University of Calgary.
The sequence demultiplexing and removal of indices were performed blindly using the bacterial metagenomics workflow in the MiSeq Reporter software (Illumina). Sequences were then processed with Mothur Version 1.35.129 following the online MiSeq standard operating procedure (https://www.mothur.org/wiki/MiSeq_SOP).30 Reads with exact barcode matching, two nucleotide mismatch in primer matching or containing ambiguous characters were removed. Very low abundant features were filtered using default options; minimum count 4 and low-count filter based on 20% prevalence in samples. To adjust for even sequencing depth, the numbers of sequences were rarefied to the same amount as the minimum number of sequence detected. Since the microbiome dataset is sparse and compositional,31 the data were transformed using centered log-ratio prior to statistical analysis. Operational taxonomic units (OTUs) were clustered based on a 97% sequence similarity against Greengenes 13_5 database. The sequence reads were further analyzed using the 16S Metagenomics app (version 1.0.1; Illumina) for taxonomy assignment. The data were transformed into centered log-ratio and used for PLS-DA analysis following the instruction by mixMC from the mixOmics R package.32 The metagenomics prediction of the 16S rRNA data were conducted using PICRUSt.33 Data from the metagenomics prediction output were also transformed into centered log-ratio and used for subsequent statistical analysis. A leave-out-out cross-validation approach was used to evaluate the PLS-DA model by MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/). Parameters including goodness of fit values R2 and accuracy value were provided. To further evaluate the model performances, we performed a linear Fisher Discriminant Analysis with stepwise variable selection (SPSS software, version 24.0; IBM, Chicago, IL, USA). Fisher Discriminant Analysis could identify the combination of predictor variables that discriminates between the control and baseline groups. Selection of predictor variables was based on Wilk's lambda and the corresponding F-statistic, entering all variables with a p-value < 0.05 into the model. F value was set at Fentry= 3.84 and Fremoval=2.71. Classification and regression analyses used the leave-one-out cross-validation to provide a statistically independent assessment of model predictions. The discriminant scores and confusion matrices from Fisher Discriminant Analysis were ploted. A random forest anlysis was performed with MicrobiomeAnalyst (https://www.microbiomeanalyst.ca). MicrobiomeAnalyst could identify important variables at different taxa level using OTU abundance tables as input datasets.
Metabolic fingerprinting with LC-QTOF-MS
Sample preparation. Sample preparation was carried out as previously described.34,35 For serum samples, protein precipitation and metabolite extraction were performed by adding 50 μL of serum to 200 μL of cold (−20 °C) 100% methanol (LC/MS grade). Samples were vortexed and centrifuged at 14000 xg for 10 min at 4 °C. The supernatant was evaporated to dryness at 45 °C in a SPD 111 V speed-vac system (Thermo Savant Instruments, Holbrook, NY, USA). The residues were washed again with 100 μL 50% methanol and centrifuged at 14000 xg for 15 min. The supernatant was filtered with 0.2 µm HPLC grade centrifugal tubes. Then 90 μL of the supernatant was collected and pooled to prepare the Quality Control (QC) samples. Hippocampal tissues (∼10 mg, wet weight) were weighed and added to 200 μL cold methanol:water (2:1). Piperazine was used as an internal standand.
Sample analysis. Samples were analyzed blindly using an Agilent 6550 iFunnel Q-TOF LC/MS system (Agilent, Santa Clara, CA, USA). Metabolite separation was performed on an ACQUITY UPLC HSS T3 column. A gradient elution with 100% water was used as mobile phase A and 100% acetonitrile as mobile phase B. Elution was started with 5% B held for 1.5 min then a linear gradient of B from 5 to 100% for 14 min, held at 100% B for 3 min. Finally, the column was re-equilibrated for 2 min. The injection volume was 2 µL for serum and 5 µL for hippocampus. Data were collected in ESI positive ionization modes. The ESI needle voltage was held at ground and the capillary voltage set at 4000 V. Source conditions were as follows: sheath gas flow, 10 L min-1; sheath gas temperature, 340 °C; nebulizer, 45 psig; drying gas temperature, 150 °C; drying gas flow, 18 L min-1; nozzle voltage, 0 V; fragmentor, 175 V. Nitrogen was used as source gas and as collision gas. Spectra were acquired over the mass range m/z 50–1000 at acquisition rate 3 spectra s-1. Reference ions for internal MS correction were introduced via the other sprayer and appear per spectra. The reference ions used were 121.050873 m/z and 922.009798 m/z for positive mode. QC samples were run every 10 samples to check the reproducibility. Raw data were converted to mzXML format by ProteoWizard 3.0 package and uploaded to XCMS online (https://xcmsonline.scripps.edu/).36 Median-fold change normalization and Pareto scaling were applied for the extracted peak abundance. Metabolites were identified against METLIN,37 Human Metabolome Database (http://www.hmdb.ca/),38 and MassBank (https://massbank.eu/). For multivariate analysis, partial least squares discriminant analysis (PLS-DA) models were obtained using SIMCA 13.0 (Umetrics, Umea, Sweden). A p-value was calculated in cross-validation analysis of variance (CV-ANOVA) to estimate the significance of the PLS model. Variable importance of projection (VIP) scores were assessed in order to rank each metabolite for their degree of discrimination within the model. The discriminated metabolites between groups were defined with a VIP > 1.0 by PLS-DA analysis, P < 0.01, FDR-ajusted p value < 0.05. The metabolic pathways were analyzed using MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/).39
Statistical analysis
The normality of data distribution was assessed with a Kolmogorov-Smirnov test. For comparisons between > 2 groups, one-way ANOVA followed by Turkey's post-hoc test (organ weight, metabolomics data, microbial diversity indices). To analyze specific microbiota changes, univariate analyses of selected species (after centered log-ratio transformation) were performed by Kruskal–Wallis ANOVA with Dunn's post-hoc test. Above tests were conducted with GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA). Data of body weight gain were analyzed using one-way ANOVA with repeated-measures and the Bonferroni post-hoc test in SPSS (SPSS version 24.0; SPSS Inc., Chicago, Illinois). Effects of diet or lesion as fixed factors were analyzed using a full-factorial general linear model ANOVA with type III sums of squares in SPSS (SPSS version 24.0; SPSS Inc., Chicago, Illinois). A false discovery rate correction was applied for multiple tests where applicable. Significant differences were indicated by *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. #p-value between 0.05 and 0.1 indicated a trend toward a significant effect. Sample size was determined to achieve a power of 0.8 for ANOVA test using the G*Power 3.1.9.2. To acquire unbiased data, the investigator who administered the treatment was the only person aware of the treatment group allocation. Another technician and research assistant evaluated the behavioral and biochemical analysis, who were blind to the types of treatments.
Ethics statement
All procedures were performed under the guidelines of the Canadian Council on Animal Care and had ethics approval by the Health Sciences Animal Care Committee at the University of Calgary (Approval ID. AC16-0271).
Role of funding source
Funders provided financial support for this study, and played no role in study design, data collection, data analyses, interpretation, or writing of the study.
Results
KD alters gut microbiota composition in the infantile spasm model
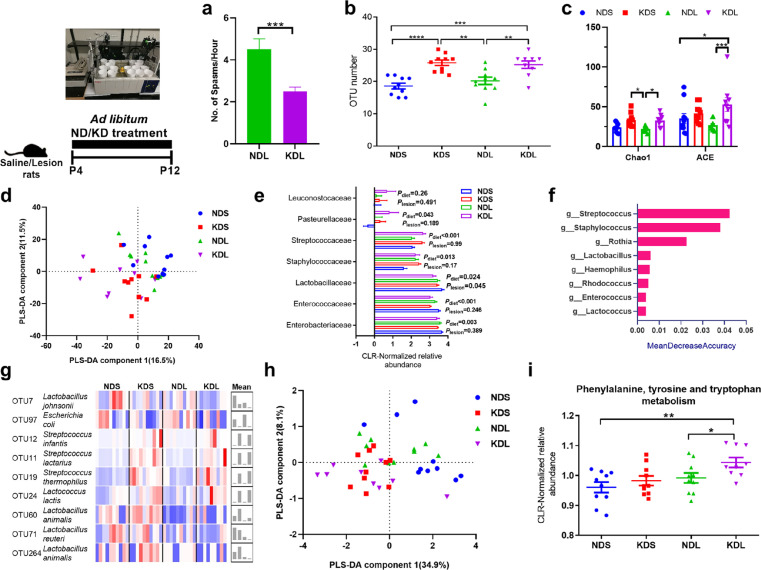
From postnatal day (P) 5–12, we observed a decrease (P < 0.001) in the number of spasms in ketogenic diet and intracerebral lesion pups (KDL) compared to normal diet and intracerebral lesion pups (NDL).40 The KD significantly reduced (P = 0.004) the number of spasms at P7 (mean±sem: 2.5±0.2) than rats received normal diet (ND; mean±sem:4.5±0.5; Figure 1a). To build on this work and explore the mechanisms by which the diet is protective, we examined the role of the gut microbiota in the fecal matter of P12 rats treated with ND or KD. A total of 151676 ± 2301 (mean ± SEM) reads were obtained per sample. KD increased alpha-diversity, as shown by higher numbers of operational taxonomic units (OTUs) (P = 0.008 for NDL vs. KDL, Figure 1b) and the Chao1 (P = 0.01 for NDL vs. KDL) and ACE indices (P < 0.001 for NDL vs. KDL) (Figure 1c). There were no differences in these measures (P = 0.674, 0.906, and 0.167 for OTU numbers, Chao1, and ACE, respectively) between rats with spasms and sham-operated control rats treated with the KD. There were also no differences in the Shannon index (measurement of community richness and evenness, Fig. S1a) between the groups (P = 0.335). The principal component analysis of microbial communities was shown as a reflection of how KD changed microbial structure (Fig. S1b). A PLS-DA model showed that the component 1 explained 16.5% of the total structural variation in gut microbiota, whereas component 2 accounted for 11.5% (Figure 1d). Samples from ND and KD were clustered separately (Figure 1d). The goodness of fit values (R2) and accuracy value were 0.86 and 0.44, respectively, in the leave-one-out cross-validation test. A Fisher Discriminant Analysis showed that 56.4% of cross-validated grouped cases correctly classified, with the confusion matrices shown in Fig. S1c. In sham-operated animals, the KD significantly changed the microbial structure (AMOVA P < 0.001). In comparison, the KD tended to change the microbial structure in lesioned animals (AMOVA P = 0.079, Table S1). By analyzing the relative abundance of bacterial taxa, we showed that lesioned pups had lower abundance of Firmicutes compared to saline-treated animals (Plesion= 0.01, Fig. S1d). We found that there were significant dietary effects of the KD on Enterococcaceae (P = 0.003), Streptococcaceae (P < 0.001) and Staphylococcaceae (P = 0.013, Figure 1e). Streptococcus, Staphylococcus, and Lactococcus were among the top important variables as showed by the random forest analysis (Figure 1f). Notably, KD increased the relative abundances of Streptococcus infantis (P = 0.001), S. lactarius (P < 0.001), S. thermophilus (P < 0.001), Lactococcus lactis (P = 0.005) and decreased Lactobacillus johnsonii (P = 0.019) and Escherichia coli (P = 0.011) compared to ND both in saline and lesioned rats (Figure 1g).
Figure 1.
The ketogenic formula changes the fecal microbiome. (a) The number of spasms. (b) The number of OTUs in saline or lesion pups fed with normal or ketogenic formula. (c) Chao1 and ACE indices. (d) PLS-DA analysis of the microbiota composition at the OTU level. (e) Respresentative microbial families that were significantly affected by diet or lesion treatment. (f) Random Forest analysis of the genus features accounting for the microbiota variation. (g) Normalized abundances of dominant OTUs in the fecal microbiome, with right histogram showing mean value per group. (h) PLS-DA analysis of the data at KEGG pathway level from metagenomics predection. (i) Alterations of phenylalanine, tyrosine and tryptophan metabolism based on the metagenomics predection. n = 10, 10, 10, 9 for NDS, KDS, NDL, and KDL, respectively. Data are presentd as mean±SEM (a, b, c, e, i). Student's t test (a), One-way ANOVA with Turkey's post-hoc test (b, c, i), full-factorial general linear model ANOVA with type III sums of squares (e): *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ND, normal diet; NDL, normal diet and intracerebral lesion; NDS, normal diet and intracerebral saline treatment; KD, ketogenic diet; KDL, ketogenic diet and intracerebral lesion; KDS, ketogenic diet and intracerebral saline treatment; OTU, operational taxonomic unit.
To further study the functional implications of the altered microbiota, we then predicted metagenomic potential using PICRUSt. The overall distribution of samples was shown in Figure 1h. The relative abundance of microbial genes related to phenylalanine, tyrosine and TRP metabolism (ko00400) was higher in lesioned rats fed KD than lesioned rats fed ND (ANOVA, P = 0.039; Figure 1i). These results clearly revealed that KD altered microbial communities in the neonatal rats.
To investigate possible contribution of the altered gut microbiota to TRP metabolism, we analyzed the presence of TRP metabolism enzymes in the bacteria affected. We searched for the presence of microbial genes affected by antibiotics employing available information in UniProt, NCBI Genome and the BRENDA database. As shown in Table S2, Lactococcus lactis species within Lactococcus can produce TRP 2,3-dioxygenase that metabolizes TRP into KYN, while both Lactococcus lactis and Streptococcus thermophilus species can produce aromatic-amino-acid transaminase that metabolizes TRP into indole-3-lactate. These species may be involved in the alteration of TRP metabolism.
KD affects metabolic status, especially TRP metabolism
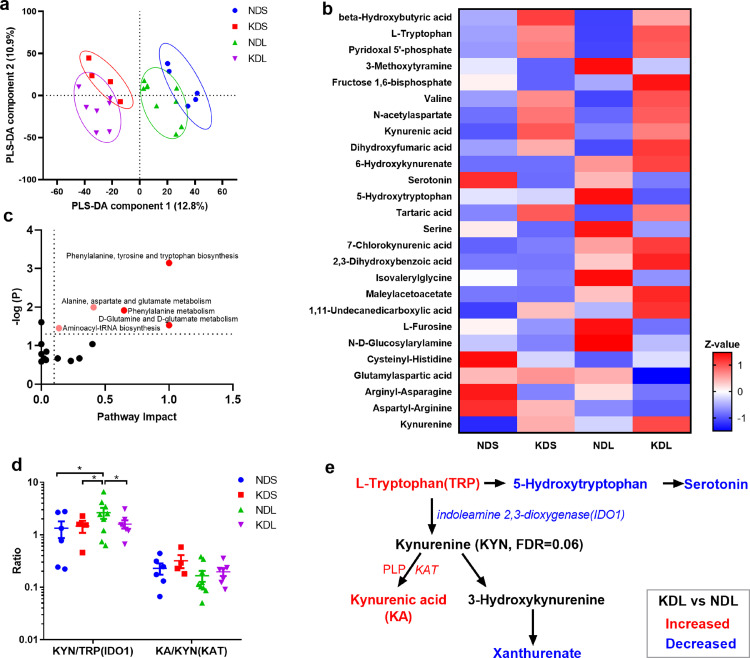
To gain further insights of the KD on TRP metabolism we performed metabolomics profiling of serum metabolites on P12 rats. We observed a distinct sample distribution of KD rats from ND rats (Figure 2a), showing the KD to change overall metabolite composition. Among the discriminant metabolites (Figure 2b, Table S3), we found higher TRP, pyridoxal 5’-phosphate, KA and lower 5-hydroxytryptophan, glutamylaspartic acid in KDL pups compared to NDL pups. Significantly changed metabolites were examined by pathway analysis, that identified the enrichment of phenylalanine, tyrosine and TRP biosynthesis (P < 0.001, Figure 2c) in KD vs ND lesioned pups. Moreover, KD affected a set of metabolites known to have anticonvulsant effects, such as β-hydroxybutyric acid,41 fructose 1,6-bisphosphate,42 and KA43 (Figure 2b). The ratio of KYN to TRP (KYN/TRP) and KA to KYN (KA/KYN) is widely used as indicators of IDO1 and KYN aminotransferase (KAT) enzyme activation, respectively.44,45 In the present study, when compared with ND saline pups (NDS), lesioned pups fed with ND had a higher (P = 0.014) KYN/TRP ratio, that was decreased by KD treatment (P = 0.039, Figure 2d). Overall, serum metabolomics analysis implicated alterations in TRP-KYN metabolism with lesion induction (Figure 2e).
Figure 2.
The ketogenic diet affects metabotype. (a) Partial least squares discriminant analysis (PLS-DA) of metabolomics pofiles. (b) Heatmap of discriminant metabolites with Variable importance of projection >1. (c) Pathway analysis of discriminant metabolites. Pathways with impact > 0.1 and p < 0.05 are highlighted. (d) The ratio of kynurenine to tryptophan (IDO1 indicator) and kynurenic acid to kynurenine (KAT indicator). (e) Summary of alterations in tryptophan metabolism after ketogenic diet treatment. n = 6, 4, 9, 7 for NDS, KDS, NDL, and KDL, respectively. Data are presentd as mean±SEM (d). One-way ANOVA followed by Turkey's post-hoc test (d): *p < 0.05. IDO1, indoleamine 2, 3-dioxygenase 1; ND, normal diet; KAT, kynurenine aminotransferase; KD, ketogenic diet; KYN, kynurenine; KA, kynurenic acid; PLP, pyridoxal phosphate; TRP, tryptophan.
Microbiota manipulation by antibiotic intervention reduces spasm frequency
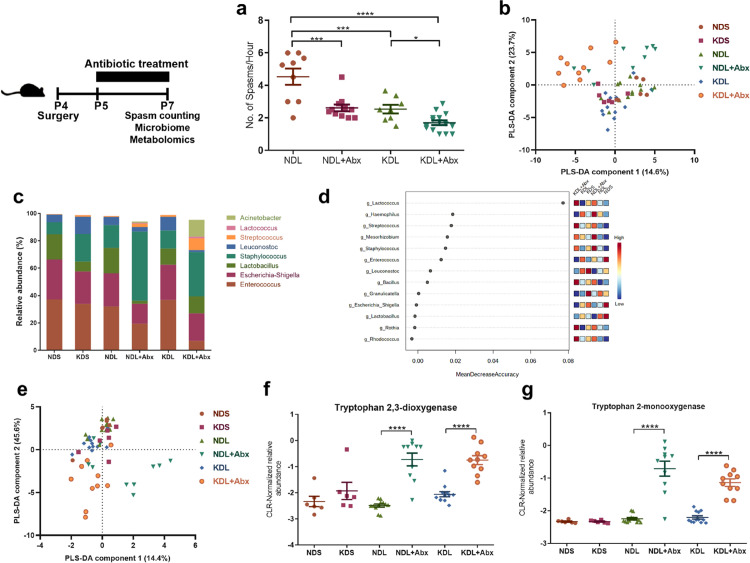
To determine if gut microbiota manipulation is related to spasm occurrence, we treated rats with a broad-spectrum antibiotic cocktail (20 mg/kg vancomycin, 50 mg/kg neomycin, 50 mg/kg ampicillin and 10 mg/kg metronidazole) administered through their formula daily for 3 days. We then focused on the spasms and microbial alterations on P7 where we have previously observed a maximum reduction of spasms by the KD.40 The 3-days treatment of KD induced ketosis in the pups (Figure S2). On P7, we noted a significant reduction in spasm frequency in lesioned rats treated with antibiotics, both in rats fed ND (P < 0.001) or KD (P = 0.039, Figure 3a). Combined treatment with oral KD and antibiotics is most effective in reducing spasm frequency, with a 2.5-fold reduction compared to ND lesioned rats. Together, these results suggest that manipulation of the gut microbiota has protective effects.
Figure 3.
Antibiotic effects on spasms frequency and fecal micribiome. (a) Number of spasms per hour. n = 8, 12, 17 15 from NDL, NDL+Abx, KDL, KDL+Abx groups, respectively. (b) PLS-DA analysis of the microbiota composition after antibiotic treatment at the OTU level. (c) Abundances of bacteria at the genus level. (d) Random Forest analysis of the genus. (e) PLS-DA analysis of the KEGG pathway data from PICRUSt analysis. (f) Alterations of the microbial tryptophan 2,3-dioxygenase. (g) Alterations of the microbial tryptophan 2-monooxygenase. (a–g): n = 6, 6, 12, 10, 12, 10 for NDS, KDS, NDL, NDL+Abx, KDL, KDL+Abx groups, respectively. Data are presentd as mean±SEM (a, f, g). One-way ANOVA followed by Turkey's post-hoc test (a), Kruskal–Wallis ANOVA with Dunn's post-hoc test (f, g): *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. NDL, normal diet and intracerebral lesion; NDS, normal diet and intracerebral saline treatment; NDL+Abx, normal diet and intracerebral lesion with oral antibiotics; KDL, ketogenic diet and intracerebral lesion; KDS, ketogenic diet and intracerebral saline treatment; KDL+Abx, ketogenic diet and intracerebral lesion with oral antibiotics.
To examine potential alterations in microbial species in response to KD and antibiotics, the fecal microbiota on P7 was profiled. A total of 117201 ± 2475 reads were obtained per sample. The antibiotics used induced a change in the microbiome composition rather than fully depleting the microbiome. Samples from antibiotic-treated animals were clustered separately from those in non-antibiotic groups as shown in the PCA (Fig. S3) and PLS-DA plot (Figure 3b). The goodness of fit values (R2) and accuracy value of PLS-DA model were 0.49 and 0.46, respectively, in the leave-one-out cross-validation test. A Fisher Discriminant Analysis showed that 64.3% of cross-validated grouped cases correctly classified, with the confusion matrices shown in Fig. S4. Antibiotic treatment significantly affected the microbial composition compared to non-antibiotic groups fed the same diet (P = 0.002 for NDL+Abx vs. NDL, P < 0.001 for KDL+Abx vs. KDL, Table S4). KDL+Abx pups also differed from NDL+Abx pups (AMOVA P = 0.013, Table S4). Firmicutes and Proteobacteria were the dominant bacteria accounting for more than 95% of total bacteria composition in all groups. At the genus level, we noted a similar effect of antibiotics in NDL or KDL rats, including higher abundances of Streptococcus (Pdiet=0.001), Lactococcus (Pdiet=0.001) and lower abundances of Lactobacillus (Pdiet= 0.016) and Enterococcus (Pdiet<0.001, Figure 3c). Streptococcus, Lactococcus, and Enterococcus were also among the top features by mean decrease accuracy in the random forest analysis (Figure 3d).
Based on the above results, we further analyzed if the IS protocol could have an impact on microbiome composition by comparing microbiome difference of NDS and NDL. On P7, NDL rats had a numerically higher Staphylococcus (P = 0.067) than NDS, no effects on diversity and other phyla (mainly Firmicutes and Proteobacteria) and genera, which means the effects were minor. On P12, the overall α-diversity was similar in terms of the number of OTUs between NDS and NDL (P = 0.28). NDL rats had higher relative abundance of Firmicutes than NDS (P = 0.043). In addition, rats were randomized to assigned for NDS and NDL. Equal diets were offered. So we suppose that the effects of IS protocol on the microbiome gradually showed up with lesion induction (probably similar at baseline).
Antibiotic intervention affects microbial and host metabolism of tryptophan
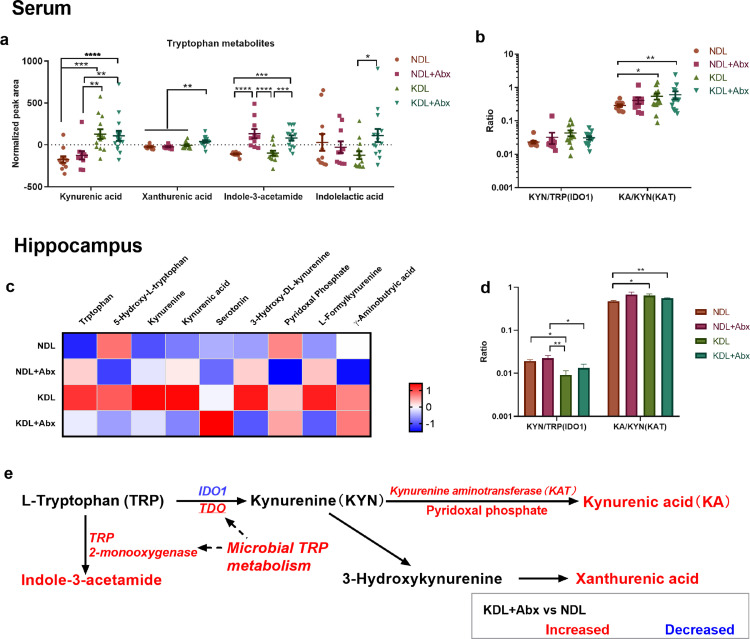
TRP metabolism is implicated in epilepsy pathogenesis.46 We investigated whether the compositional alterations by antibiotics were reflected in microbial TRP metabolism. Samples from antibiotic-treated animals were clustered separately from those in non-antibiotic groups as shown in the PLS-DA plot (Figure 3e). Microbial functional prediction analysis using PICRUSt found an increase in KEGG Orthology related to TRP 2,3-dioxygenase (P < 0.001 for NDL vs. NDL+Abx, P < 0.001 for KDL vs. KDL+Abx) and TRP 2-monooxygenase (P < 0.001 for NDL vs. NDL+Abx, P < 0.001 for KDL vs. KDL+Abx) by antibiotic treatment (Figure 3f and g). Supporting these results, the microbial metabolite indole-3-acetamide produced by TRP 2-monooxygenase, was increased in the serum of Abx rats (P < 0.001 for both NDL vs. NDL+Abx and KDL vs. KDL+Abx, Figure 4a).
Figure 4.
Antibiotics affect tryptophan-kynurenine metabolism. (a) Relative concentration of tryptophan metabolites in serum. (b) The ratio of kynurenine to tryptophan (IDO1 indicator) and kynurenic acid to kynurenine (KAT indicator) from serum profiles. n = 11, 10, 13, 14 for NDL, NDL+Abx, KDL, KDL+Abx groups, respectively (a, b). (c) Heatmap of discriminant metabolites in hippocampus. (d) The ratio of kynurenine to tryptophan and kynurenic acid to kynurenine in the hippocampal profiles. n = 13, 14, 14, 14 for NDL, NDL+Abx, KDL, KDL+Abx groups, respectively (C, D). (e) Summary of antibiotic effects on metabolism. Data are presentd as mean±SEM (a, b, d). One-way ANOVA followed by Turkey's post-hoc test (a, b, d): *p < 0.05. NDL, normal diet and intracerebral lesion; NDL+Abx, normal diet and intracerebral lesion with oral antibiotics; KDL, ketogenic diet and intracerebral lesion; KDL+Abx, ketogenic diet and intracerebral lesion with oral antibiotics; IDO1, indoleamine 2, 3-dioxygenase 1; KAT, kynurenine aminotransferase; KYN, kynurenine; KA, kynurenic acid; PLP, pyridoxal phosphate; TRP, tryptophan.
The relative concentrations of KA and xanthurenic acid were increased in the serum of KDL+Abx rats compared to NDL (P < 0.001, Figure 4a). Compared to NDL pups, KDL (P = 0.036) and KDL+Abx (P = 0.009) had an increase in the KA/KYN ratio in serum (Figure 4b), indicating an increase in KAT activity. In the hippocampus, KDL+Abx tended to increase (P = 0.071) the relative concentration of γ-aminobutyric acid compared to NDL+Abx (Figure 4c). Meanwhile, KDL pups had lower ratio of KYN/TRP (indicator of IDO1 activity; P = 0.018) and higher KA/KYN ratio (P = 0.036) than NDL pups (Figure 4d), demonstrating an alteration of TRP metabolism toward KA production (Figure 4e).
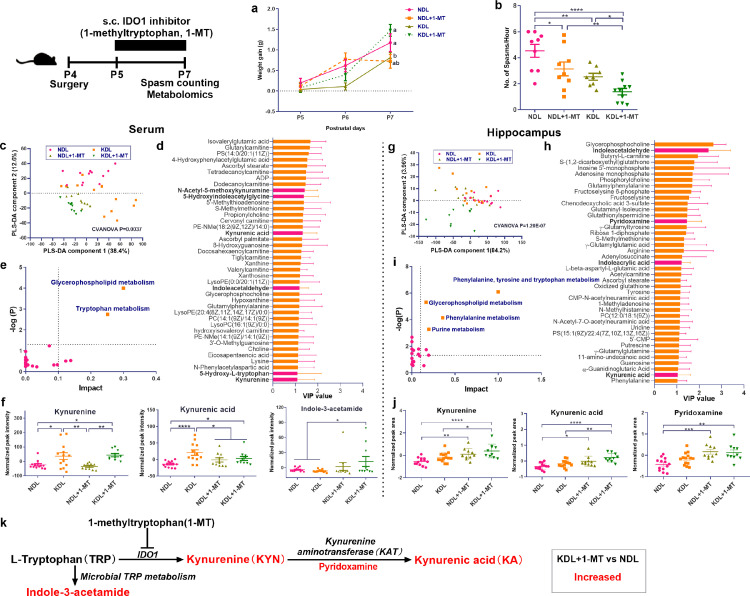
Inhibitor of TRP metabolism, IDO1 reduces spasm frequency
IDO1 is a key enzyme in TRP catabolism, determining the fate of the amino acid.8 As determined in the aforementioned results, lesioned pups fed with ND had a higher IDO1 ratio than saline pups, which was lowered after KD treatment, indicating a potential mechanism whereby reduced IDO1 may underlie the anticonvulsant effects of KD. To test this hypothesis, we used a specific IDO1 antagonist, 1-methyltryptophan (1-MT), to test if IDO1 inhibition affected spasms frequency. Compared with KDL pups, NDL (P = 0.020) and KDL+1-MT (P = 0.023) pups had higher body weight gain (Figure 5a). Compared with NDL pups, NDL+1-MT (P = 0.022) or KD+1-MT (P < 0.001) reduced spasms frequency (Figure 5b). We then profiled the serum and hippocampus metabolites to gain metabolic insights into the improvement of spasm by 1-MT treatment. Analysis of serum metabolomics profiles showed distinct metabolite compositions after 1-MT (CVANOVA P = 0.004), as visualized by the separated cluster of samples in the PLS-DA scatter plot (Figure 5c). Among the discriminant metabolites with VIP > 1, we identified metabolites involved in TRP metabolism (KA, 5-HT, KYN, etc) and mitochondrial metabolism (ADP, dodecanoylcarnitine, etc.) (Figure 5d). The pathway analysis of the discriminated metabolites identified significant alterations in TRP metabolism (P = 0.002, pathway impact > 0.1, Figure 5e). Interestingly, compared with NDL pups, KD alone or in combination with 1-MT treatment increased the relative concentration of KYN (P = 0.023 and 0.017, respectively) and KA (P < 0.001 and 0.012, respectively) in serum, and KDL+1-MT also increased (P = 0.035) indole-3-acetamide (Figure 5f), a microbial derived indole metabolite.
Figure 5.
Effects of pharmacological inhibitor of IDO1 on body weight, spasms frequency, and metabotype. (a) Body weight gain in pups treated with or without 1-methyltryptphan. n = 14, 10, 20, 15 for NDL, NDL+1-MT, KDL, KDL+1-MT groups, respectively. (b) Spasm frequency in pups treated with or without 1-methyltryptphan. n = 8, 9, 17, 10 for NDL, NDL+1-MT, KDL, KDL+1-MT groups, respectively. (c) Partial least squares discriminant analysis of serum metabolomics pofiles. (d) Discriminant metabolites in serum. (e) Pathway analysis of serum discriminant metabolites. (f) Representative metabolites involved in kynurenine metabolism from serum profiles. n = 10–13 (c–f). (g) Partial least squares discriminant analysis of hippocampal metabolomics pofiles. (h) Discriminant metabolites in hippocampus. (i) Pathway analysis of hippocampal discriminant metabolites. (j) Representative metabolites involved in kynurenine metabolism from hippocampal profiles. n = 10–14 (G–J). (k) Summary of trypophan metabolism affected by 1-MT treatment. Data are presentd as mean±SEM. One-way ANOVA with repeated-measures and Bonferroni post hoc test (a): different letters indicate significant difference, p < 0.05. One-way ANOVA followed by Turkey's post-hoc test (b, f, j): *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. IDO1, indoleamine 2, 3-dioxygenase 1; KAT, kynurenine aminotransferase; NDL, normal diet and intracerebral lesion; KDL, ketogenic diet and intracerebral lesion.
In the hippocampus, metabolomics profiles were also distinct across groups (CVANOVA P = 1.20E-07, Figure 5g). Discriminant metabolites involved in TRP metabolism were also identified in the hippocampus, such as indoleacetaldehyde, pyrodoxamine, and KA (Figure 5h). Pathway analysis indicated the alteration of TRP metabolism (Figure 5i). Compared with NDL group, 1-MT treatment with ND or KD increased the relative concentration of KYN (P = 0.002 and P < 0.001, respectively), KA (P = 0.019 and P < 0.001, respectively), and pyridoxamine (P < 0.001 and P = 0.001, respectively), a cofactor involved in KYN metabolism (Figure 5j), as also summarized in Figure 5k. Since KA is a known anticonvulsant metabolite, these results implicate the potential role of KA in mediating the anticonvulsant effects of 1-MT inhibition.
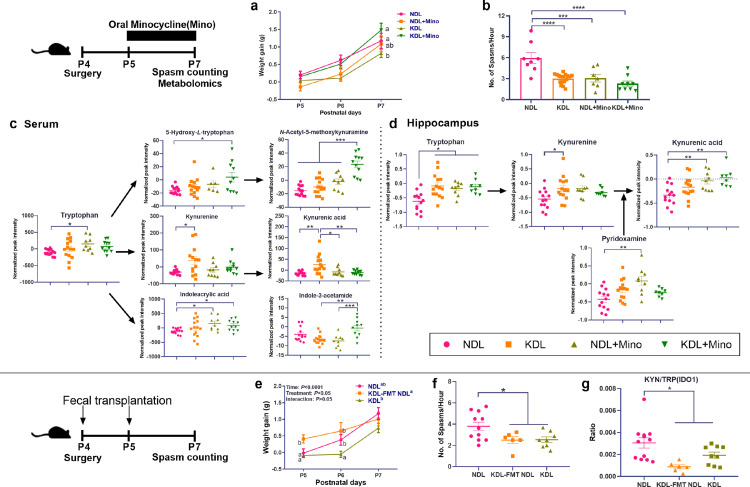
To validate the IDO1 inhibition hypothesis, we used another alternative IDO1 inhibitor, minocycline.47 KD and minocycline combination also increased body weight gain compared to KDL pups (P = 0.014, Figure 6a). The frequency of spasms was reduced in NDL+minocycline (P < 0.001) and KDL+ minocycline (P < 0.001) pups compared to NDL pups, while no difference was found between KDL and KDL+Minocycline pups (P = 0.563, Figure 6b). To investigate the potential metabolism-related mechanism, we then profiled the metabolites in the serum and hippocampus. Minocycline treatment led to distinct metabolite profiles both in NDL and KDL pups (CVANOVA P < 0.001) (Fig. S5a), such as the changed glycerophospholipid metabolites (PS(6:0/6:0), PC(0:0/5:0)) and vitamin B6 metabolites (pyridoxamine) (Fig. S5b and S5c). Among TRP metabolites, NDL+minocycline (P = 0.044) and KDL+minocycline (P = 0.206) tended to increase TRP relative to NDL groups (Figure 6c). KDL+minocycline pups had higher relative abundances of metabolites in serotonin pathway (5-hydroxy-L-TRP [P = 0.014], N-acetyl-5-methoxykynuramine[P < 0.001]), and microbial metabolite indoleacrylic acid (P = 0.046) than NDL pups (Figure 6c). In the hippocampus, a different sample distribution of minocycline-treated pups was also observed (CVANOVA P < 0.001, Fig. S5d), mainly due to the change of metabolites involved in mitochondrial metabolism (adenosine monophosphate, acetylcarnitine) and TRP metabolism (KA, 6-Hydroxymelatonin) (Fig. S5e and S5f). Among TRP metabolites, NDL+minocycline and KDL+minocycline increased KA compared to NDL (P = 0.009 and 0.002, respectively) in the hippocampus (Figure. 6d). Overall, both 1-MT and minocycline shifted TRP metabolism toward KA production in the hippocampus that may be involved in the anticonvulsant effects.
Figure 6.
Effects of minocycline and fecal microbiome transplantation on spasms. (a) Body weight gain in pups treated with or without minocycline. (b) Effects of minocycline on spasm frequency in pups. n = 8, 7, 14, 9 for NDL, NDL+minocycline, KDL, KDL+minocycline groups, respectively. (c) Effects of minocycline on trypophan metabolites in serum. (d) Effects of minocycline on trypophan metabolites in hippocampus. n = 9–14 (c, d). (e) Effects of fecal microbiome transplantation on body weight gain in pups. (f) Effects of fecal microbiome transplantation on spasm frequency in pups. (g) Ratio of KYN/TRP. n = 11, 6, 8, 9 for NDL, NDL+FMT, KDL groups, respectively (e, f, g). Data are presentd as mean±SEM. One-way ANOVA with repeated-measures and Bonferroni post hoc test (a, e): different letters indicate significant difference, p < 0.05. One-way ANOVA followed by Turkey's post-hoc test (b, c, d, f, g): *p < 0.05, **p < 0.01, ***p < 0.001. Mino, minocycline; FMT, fecal microbiota transplantation.
Effects of fecal microbiota transplantation on spasms occurrence
To further investigate whether direct microbiota manipulation affects spasms, we performed a pilot fecal microbiota transplantation trial administering fecal microbiota slurry from KDL pups to NDL pups. Compared with NDL pups, the pups receiving microbiota transplantation had higher body weight although not significant during the study (P = 0.555, Figure 6e). However, microbiota transplantation in NDL pups significantly reduced (P = 0.023) the frequency of spasm to the level similar with lesioned pups fed KD, all of which were lower than NDL pups (Figure 6f). The ratio of KYN/TRP was lower in the microbiota transplanted pups than NDL (P = 0.002, Figure 6g), which indicates the possibility of using microbiota transplantation in the context of anticonvulsant studies.
Discussion
In the present study, we demonstrated that manipulations affecting TRP metabolism, possibly via the gut microbiota, altered the occurrence of IS. Employing a rodent model of IS that recapitulates key features of the condition, we observed that antibiotics worked on their own to reduce spasms and improve the effectiveness of the KD when given in combination, which was associated with changes in TRP-KYN metabolism. Pharmacological inhibition of IDO1 increased KA production and was neuroprotective in the IS model. Thus a combination of KD with antibiotics or IDO1 inhibition may offer an alternative therapy in IS. Finally, we also demonstrated that fecal microbiota transplant from specific bacterial species induced by the KD provided protection against spasms.
Interestingly, the KD increased the relative abundance of Lactococcus and Streptococcus, microbes that are capable of metabolizing TRP that were further increased after antibiotics treatment. Species within Lactococcus have high capability to metabolize cholesterol and tolerate antibiotics such as neomycin, which may give rise to the increase of these species.48 Whether specific bacterial strains or their metabolites directly contribute to spasm amelioration remains unknown. Nevertheless, fecal transplant from lesioned rats treated with the KD to untreated lesioned rats produced anti-spasms effects which indicates that the gut microbiota is involved in the mechanism of action of the KD in IS which we postulate is due to a microbiota-mediated effect on TRP metabolism.
Analysis of the gut microbiota revealed an increase in the Chao1 and ACE indices, an estimate of species richness and α-diversity, after the ketogenic diet. This finding is discordant with other studies showing either no change in α-diversity in children aged 2.2-15.3 years,20 or a decrease in α-diversity with the diet in adult mice or children aged 1.2-10.3 years.49 Firmicutes and Bacteroidetes are dominant in the gut microbiota of these subjects. In the present study, the neonatal microbiota is premature with dominance of Firmicutes and Proteobacteria. The reason for the discordance in alpha diversity may be due to the fact that the spasms are induced in neonatal rats at an age when the microbiota is still developing. An effect of the artificial rearing method or epilepsy model type also has not been excluded.
Results of the present study suggest that the gut microbiota may mediate spasm frequency via TRP metabolism, changes in circulating microbial indole derivatives and KYN metabolites. It is well documented that the gut microbiota is a major factor influencing TRP metabolism.50 Gut microbes encode enzymes metabolizing TRP into 5-HT, KYN and indole metabolites affecting TRP availability in vivo.50,51 In the KDL pups receiving antibiotics, microbial KYN metabolism was enhanced and indole derivatives such as indole-3-acetamide and indolelactic acid were increased in circulation, indicating the possible contribution of microbial TRP metabolism by the gut. It is also worth noting that the identified indole derivatives are precursors of aryl hydrocarbon receptor ligands, molecules known to exert anti-inflammatory activity.52,53 From the present study, we cannot rule out the possibility that the anti- spasms effects of antibiotics are mediated through immune mediators as upregulation of inflammatory neuropathology is involved in epilepsy in early childhood.54
The use of antibiotics in epilepsy treatment is controversial. In a clinical report, children with drug-resistant epilepsy who received individual-specific antibiotic treatment (e.g. amoxicillin/clavulanic acid or clarithromycin) exhibited less frequent seizures in 5 children who were taking anti-seizure medications (e.g. valproic acid, clobazam, levetiracetam) and in 1 child being treated with both anti-seizure medications and the ketogenic diet. The beneficial effects of antibiotics were eliminated after their cessation.55 One of the tetracyclines, minocycline also reduced seizure frequency in a rat model of pilocarpine-induced status epilepticus.56 In the present study, KD in combination with clinically relevant antibiotic combinations (vancomycin, neomycin, ampicillin and metronidazole) reduced spasm frequency. However, antibiotics such as penicillin and cephalosporins, have the opposite effects, inducing seizures and interfering with the activity of anti-seizure medications.57 Reasons for this discrepancy are unknown, but may be due to a complex interplay between the direct effects of antibiotics on cerebral excitability58 and indirectly through the gut microbiota as our results indicate.
Another finding is the overall trend toward an increase in the KA/KYN ratio (KAT indicator) within the circulation and hippocampus after KD or KD+Abx treatment, in comparison to the NDL group, possibly leading to higher levels of circulating KA. Metabolism of KYN into KA by KAT may be compromised in IS, as earlier studies have found lower KA in the cerebrospinal fluid of IS patients.46,59 The KYN hypothesis, namely low production of KA from KYN, has been proposed in IS pathogenesis.9 In support of this hypothesis, enhancement of brain KA production has been found to regulate epileptogenesis in adult rats.60,61 A recent study further verified that KD intervention increased the concentration of KA, decreased KYN and seizure frequency in patients with refractory epilepsy.62
In the present study, the KDS and KDL rat pups showed similar values of KYN/TRP ratio. However, the NDL showed increased KYN/TRP ratio relative to NDS, indicating altered tryptophan metabolism after lesion induction. Consistent with this finding, a recent study by Deng and colleagues63 also reported the increased KYN/TRP ratio in the serum of epilepsy patients. The elevated KYN/TRP ratio in NDL rats is likely due to the induction of spasms which is normalized by the KD.
We further showed that IDO1 inhibition by 1-methyltryptophan or minocycline reduces spasms frequency, a finding that implicates KA in seizure susceptibility. IDO1 attracted our attention for two reasons. First, the association between IDO1 and neurodevelopmental disorder is well-established.64 Second, this pathway was significantly decreased following gut microbiota manipulation. IDO1 activation has been shown to promote co-morbid depression in mice with chronic temporal lobe epilepsy,65 while IDO1 inhibition ameliorated depression-like behaviors.25,65 In addition, IDO1 inhibition also ameliorated neuropathic pain in adult rats induced by intrathecal catheter implantation.66 Consistent with the altered levels of KA in infantile spasms, inhibition of TRP-2,3-dioxygenase (functionally homologous to IDO1) increased KA formation, ameliorated neurodegeneration and improved locomotor performance in fruit fly models of Alzheimer's, Parkinson's, and Huntington's diseases.64 Overall, these findings indicate that IDO1 may serve as a target for treating intractable infantile spasms.
Based on the current findings, we can not rule out the microbiome-independent effects of antibiotics on seizure protection. Neomycin and vancomycin are non-absorbable antibiotics incapable of crossing the blood-brain barrier and become concentrated in the gut. Some antibiotics have direct neuronal impacts. For instance, ampicillin could regulate the expression of glutamate transporter EAAT2 that is neuroprotective in organotypic spinal cord slice cultures from rats.67 Upregulation of glutamate transporter in astrocyte has been shown to reduce seizure frequency in a mouse model of temporal lobe epilepsy induced by intrahippocampal kainic acid.68 This is interesting and could be examined in future studies. Another limitation of the present study is that the mechanism of FMT in reducing spasms remains unclear. It is unknown whether the reduced spasms after FMT is due to alterations in microbiome composition or residual dietary molecules from the KD itself. Due to the pilot nature of the FMT study, we have not profiled the microbiome composition after FMT. It is one of the future interests to expand the dataset from the FMT trial. Additionally, due to the naïve nature of the newborn pups, the stool was quite tiny < 10 mg per pup. To yield enough amount of DNA for sequencing, several stool samples were merged. In doing so, the samples used for microbiome and metabolome were not corresponding to each other, which greatly hindered a correlation analysis based on microbiome-metabolome data.
In conclusion, our study reveals that KD and/or antibiotics change the gut microbiota and reduce spasm severity in a symptomatic rat model of IS. In addition, transplant of faeces from lesioned pups fed the KD to lesioned pups fed the normal milk diet reduced the frequency of spasms to a level similar to KD fed animals. Our results show strong evidence that these effects of the gut microbiota on IS are mediated by pathways involving TRP metabolism. Future studies are needed to determine whether the gut microbiota can be manipulated in the clinic to mitigate spasms frequency, possibly by the administration of specific probiotics.
Declaration of interests
Dr Chunlong Mu received an Alberta Children's Hospital Research Institute Trainee Travel Award which provided payment for hotel and travel for research meeting. No non-financial conflicts of interest exist for any of the authors.
Acknowledgments
Contributors
CM performed experiments, analyzed data, and wrote the manuscript. AC, SM, and KTB performed experiments and edited the manuscript. CM, JMR, JS, and MHS conceived the study and are responsible for the experimental design and manuscript preparation; C.M. and J.S. has directly accessed and verified the underlying data reported in the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgments
This study was supported by the funding from the Alberta Children's Hospital Research Institute and the Owerko Centre. We thank the technical support on HPLC Q-TOF MS from ACH-BioCORE lab funded by the University of Calgary, Alberta Health Services, and the Alberta Children's Hospital Foundation.
Data sharing statement
The sequencing data is available under PRJNA771924 within NCBI Sequence Read Archive. All supporting data are included in the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2022.103833.
Appendix. Supplementary materials
References
- 1.Hrachovy R.A., Frost J.D. Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome) J Clin Neurophysiol. 2003;20(6):408–425. doi: 10.1097/00004691-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Riikonen R. Infantile spasms: outcome in clinical studies. Pediatr Neurol. 2020;108:54–64. doi: 10.1016/j.pediatrneurol.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Go C.Y., Mackay M.T., Weiss S.K., et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the guideline development subcommittee of the american academy of neurology and the practice committee of the child neurology society. Neurology. 2012;78(24):1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower B.D. The tryptophan load test in the syndrome of infantile spasms with oligophrenia. Proc R Soc Med. 1961;54(7):540–544. [PMC free article] [PubMed] [Google Scholar]
- 5.Langlais P.J., Wardlow M.L., Yamamoto H. Changes in CSF neurotransmitters in infantile spasms. Pediatr Neurol. 1991;7(6):440–445. doi: 10.1016/0887-8994(91)90028-j. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H., Egawa B., Horiguchi K., Kaku A., Yamada K. [Changes in CSF tryptophan metabolite levels in infantile spasms] No To Hattatsu. 1992;24(6):530–535. [PubMed] [Google Scholar]
- 7.Silverstein F., Johnston M.V. Cerebrospinal fluid monoamine metabolites in patients with infantile spasms. Neurology. 1984;34(1):102–105. doi: 10.1212/wnl.34.1.102. [DOI] [PubMed] [Google Scholar]
- 8.Muneer A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: pathophysiologic and therapeutic considerations. Clin Psychopharmacol Neurosci. 2020;18(4):507–526. doi: 10.9758/cpn.2020.18.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rho J.M. Basic science behind the catastrophic epilepsies. Epilepsia. 2004;45(Suppl 5):5–11. doi: 10.1111/j.0013-9580.2004.05001.x. [DOI] [PubMed] [Google Scholar]
- 10.Lapin I.P. Convulsions and tremor in immature rats after intraperitoneal injection of kynurenine and its metabolites. Pharmacol Res Commun. 1978;10(1):81–84. doi: 10.1016/s0031-6989(78)80065-4. [DOI] [PubMed] [Google Scholar]
- 11.Lapin I.P. Kynurenines and seizures. Epilepsia. 1981;22(3):257–265. doi: 10.1111/j.1528-1157.1981.tb04108.x. [DOI] [PubMed] [Google Scholar]
- 12.Hong A.M., Turner Z., Hamdy R.F., Kossoff E.H. Infantile spasms treated with the ketogenic diet: prospective single-center experience in 104 consecutive infants. Epilepsia. 2010;51(8):1403–1407. doi: 10.1111/j.1528-1167.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang H.C., Lee Y.J., Lee J.S., et al. Comparison of short- versus long-term ketogenic diet for intractable infantile spasms. Epilepsia. 2011;52(4):781–787. doi: 10.1111/j.1528-1167.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- 14.Eun S.H., Kang H.C., Kim D.W., Kim H.D. Ketogenic diet for treatment of infantile spasms. Brain Dev. 2006;28(9):566–571. doi: 10.1016/j.braindev.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Heischmann S., Gano L.B., Quinn K., et al. Regulation of kynurenine metabolism by a ketogenic diet. J Lipid Res. 2018;59(6):958–966. doi: 10.1194/jlr.M079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth W., Zadeh K., Vekariya R., Ge Y., Mohamadzadeh M. Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci. 2021;22(6) doi: 10.3390/ijms22062973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao K., Mu C.L., Farzi A., Zhu W.Y. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. 2020;11(3):709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano J.M., Yu K., Donaldson G.P., et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng A.J., Qiu X.M., Lai W.L., et al. Altered composition of the gut microbiome in patients with drug-resistant epilepsy. Epilepsy Res. 2018;147:102–107. doi: 10.1016/j.eplepsyres.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Lindefeldt M., Eng A., Darban H., et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes. 2019;5(1):1–13. doi: 10.1038/s41522-018-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie G., Zhou Q., Qiu C.Z., et al. Ketogenic diet poses a significant effect on imbalanced gut microbiota in infants with refractory epilepsy. World J Gastroenterol. 2017;23(33):6164–6171. doi: 10.3748/wjg.v23.i33.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y.J., Zhou S.Z., Zhou Y.F., Yu L.F., Zhang L.M., Wang Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018;145:163–168. doi: 10.1016/j.eplepsyres.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Scantlebury M.H., Galanopoulou A.S., Chudomelova L., Raffo E., Betancourth D., Moshe S.L. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scantlebury M.H., Galanopoulou A.S., Chudomelova L., Raffo E., Betancourth D., Moshe S.L. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37(3):604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza L.C., Jesse C.R., de Gomes M.G., et al. Activation of brain indoleamine-2,3-dioxygenase contributes to depressive-like behavior induced by an intracerebroventricular injection of streptozotocin in mice. Neurochem Res. 2017;42(10):2982–2995. doi: 10.1007/s11064-017-2329-2. [DOI] [PubMed] [Google Scholar]
- 26.Beheshti Nasr S.M., Moghimi A., Mohammad-Zadeh M., Shamsizadeh A., Noorbakhsh S.M. The effect of minocycline on seizures induced by amygdala kindling in rats. Seizure. 2013;22(8):670–674. doi: 10.1016/j.seizure.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Kumar H., Sharma B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Res. 2016;1630:83–97. doi: 10.1016/j.brainres.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 28.Klindworth A., Pruesse E., Schweer T., et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schloss P.D., Westcott S.L., Ryabin T., et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozich J.J., Westcott S.L., Baxter N.T., Highlander S.K., Schloss P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79(17):5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gloor G.B., Macklaim J.M., Pawlowsky-Glahn V., Egozcue J.J. Microbiome datasets are compositional: and this is not optional. Front Microbiol. 2017;8:2224. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohart F., Gautier B., Singh A., Le Cao K.A. mixOmics: an R package for 'omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11):e1005752. doi: 10.1371/journal.pcbi.1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langille M.G.I., Zaneveld J., Caporaso J.G., et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai Y.S., Chen W.C., Kuo T.C., et al. Mass-spectrometry-based serum metabolomics of a C57BL/6J mouse model of high-fat-diet-induced non-alcoholic fatty liver disease development. J Agric Food Chem. 2015;63(35):7873–7884. doi: 10.1021/acs.jafc.5b02830. [DOI] [PubMed] [Google Scholar]
- 35.Southam A.D., Weber R.J., Engel J., Jones M.R., Viant M.R. A complete workflow for high-resolution spectral-stitching nanoelectrospray direct-infusion mass-spectrometry-based metabolomics and lipidomics. Nat Protoc. 2016;12(2):310–328. doi: 10.1038/nprot.2016.156. [DOI] [PubMed] [Google Scholar]
- 36.Tautenhahn R., Patti G.J., Rinehart D., Siuzdak G. XCMS online: a web-based platform to process untargeted metabolomic data. Anal Chem. 2012;84(11):5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith C.A., O'Maille G., Want E.J., et al. METLIN-a metabolite mass spectral database. Ther Drug Monit. 2005;27(6):747–751. doi: 10.1097/01.ftd.0000179845.53213.39. [DOI] [PubMed] [Google Scholar]
- 38.Wishart D.S., Jewison T., Guo A.C., et al. HMDB 3.0-the human metabolome database in 2013. Nucleic Acids Res. 2013;41(D1):D801–D8D7. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong J., Soufan O., Li C., et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486–WW94. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhary A., Mu C., Barrett K., et al. The link between brain acidosis, breathing, and seizures: a novel mechanism of action for the ketogenic diet in a model of infantile spasms. Brain Commun. 2021;3(4) doi: 10.1093/braincomms/fcab189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D.Y., Simeone K.A., Simeone T.A., et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78(1):77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lian X.Y., Khan F.A., Stringer J.L. Fructose-1,6-bisphosphate has anticonvulsant activity in models of acute seizures in adult rats. J Neurosci. 2007;27(44):12007–12011. doi: 10.1523/JNEUROSCI.3163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foster A.C., Vezzani A., French E.D., Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984;48(3):273–278. doi: 10.1016/0304-3940(84)90050-8. [DOI] [PubMed] [Google Scholar]
- 44.Boros F.A., Klivenyi P., Toldi J., Vecsei L. Indoleamine 2,3-dioxygenase as a novel therapeutic target for Huntington's disease. Expert Opin Ther Targets. 2019;23(1):39–51. doi: 10.1080/14728222.2019.1549231. [DOI] [PubMed] [Google Scholar]
- 45.Zimmer P., Schmidt M.E., Prentzell M.T., et al. Resistance exercise reduces kynurenine pathway metabolites in breast cancer patients undergoing radiotherapy. Front Oncol. 2019;9 doi: 10.3389/fonc.2019.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellstrom B., Vassella F. Tryptophan metabolism in infantile spasm. Acta Paediatr Scand. 1962;51(6):665–673. doi: 10.1111/j.1651-2227.1962.tb06599.x. [DOI] [PubMed] [Google Scholar]
- 47.Singh T., Goel R.K. Adjuvant indoleamine 2,3-dioxygenase enzyme inhibition for comprehensive management of epilepsy and comorbid depression. Eur J Pharmacol. 2016;784:111–120. doi: 10.1016/j.ejphar.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Pelinescu D., Chifiriuc M.C., Ditu L.M., et al. Selection and characterization of the probiotic potential of some lactic acid bacteria isolated from infant feces. Rom Biotechnol Lett. 2011;16(3):6278–6289. [Google Scholar]
- 49.Paoli A., Mancin L., Bianco A., Thomas E., Mota J.F., Piccini F. Ketogenic diet and microbiota: friends or enemies? Genes. 2019;10(7):534. doi: 10.3390/genes10070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Waclawikova B., El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals. 2018;11(3) doi: 10.3390/ph11030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wlodarska M., Luo C., Kolde R., et al. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22(1):25–37. doi: 10.1016/j.chom.2017.06.007. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelante T., Iannitti R.G., Fallarino F., et al. Tryptophan feeding of the IDO1-AhR axis in host–microbial symbiosis. Front Immunol. 2014;5:640. doi: 10.3389/fimmu.2014.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarnat H.B., Scantlebury M.H. Novel inflammatory neuropathology in immature brain: (1) fetal tuberous sclerosis, (2) febrile seizures, (3) alpha-b-crystallin, and (4) role of astrocytes. Semin Pediatr Neurol. 2017;24(3):152–160. doi: 10.1016/j.spen.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Braakman H.M.H., van Ingen J. Can epilepsy be treated by antibiotics? J Neurol. 2018;265(8):1934–1936. doi: 10.1007/s00415-018-8943-3. [DOI] [PubMed] [Google Scholar]
- 56.Wang N., Mi X., Gao B., et al. Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience. 2015;287:144–156. doi: 10.1016/j.neuroscience.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 57.Sander J.W., Perucca E. Epilepsy and comorbidity: infections and antimicrobials usage in relation to epilepsy management. Acta Neurol Scand Suppl. 2003;180:16–22. doi: 10.1034/j.1600-0404.108.s180.3.x. [DOI] [PubMed] [Google Scholar]
- 58.Tsuda A., Ito M., Kishi K., Shiraishi H., Tsuda H., Mori C. Effect of penicillin on GABA-gated chloride ion influx. Neurochem Res. 1994;19(1):1–4. doi: 10.1007/BF00966719. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto H., Shindo I., Egawa B., Horiguchi K. Kynurenic acid is decreased in cerebrospinal-fluid of patients with infantile spasms. Pediatr Neurol. 1994;10(1):9–12. doi: 10.1016/0887-8994(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 60.Carpenedo R., Chiarugi A., Russi P., et al. Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience. 1994;61(2):237–244. doi: 10.1016/0306-4522(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 61.Kocki T., Wielosz M., Turski W.A., Urbanska E.M. Enhancement of brain kynurenic acid production by anticonvulsants-novel mechanism of antiepileptic activity? Eur J Pharmacol. 2006;541(3):147–151. doi: 10.1016/j.ejphar.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Zarnowska I., Wrobel-Dudzinska D., Tulidowicz-Bielak M., et al. Changes in tryptophan and kynurenine pathway metabolites in the blood of children treated with ketogenic diet for refractory epilepsy. Seizure. 2019;69:265–272. doi: 10.1016/j.seizure.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Deng N., Hu J., Hong Y., et al. Indoleamine-2,3-dioxygenase 1 deficiency suppresses seizures in epilepsy. Front Cell Neurosci. 2021;15:638854. doi: 10.3389/fncel.2021.638854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Breda C., Sathyasaikumar K.V., Sograte Idrissi S., et al. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci USA. 2016;113(19):5435–5440. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie W., Cai L., Yu Y., et al. Activation of brain indoleamine 2,3-dioxygenase contributes to epilepsy-associated depressive-like behavior in rats with chronic temporal lobe epilepsy. J Neuroinflamm. 2014;11:41. doi: 10.1186/1742-2094-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rojewska E., Ciapala K., Piotrowska A., Makuch W., Mika J. Pharmacological inhibition of indoleamine 2,3-dioxygenase-2 and kynurenine 3-monooxygenase, enzymes of the kynurenine pathway, significantly diminishes neuropathic pain in a rat model. Front Pharmacol. 2018;9:724. doi: 10.3389/fphar.2018.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothstein J.D., Patel S., Regan M.R., et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 68.Peterson A.R., Garcia T.A., Cullion K., Tiwari-Woodruff S.K., Pedapati E.V., Binder D.K. Targeted overexpression of glutamate transporter-1 reduces seizures and attenuates pathological changes in a mouse model of epilepsy. Neurobiol Dis. 2021;157:105443. doi: 10.1016/j.nbd.2021.105443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.