Abstract
Objectives
Bempedoic acid (BA) is a novel oral low-density lipoprotein cholesterol lowering drug. This systematic review and meta-analysis aims to assess efficacy and safety for clinical outcomes in high cardiovascular (CV) risk patients.
Data sources
MEDLINE, Cochrane Central Register of Controlled Trials, Google Scholar, Embase, ClinicalTrials.gov, Clinical Trial Results and the American College of Cardiology web site were searched.
Study selection
Randomised controlled trials (RCTs) of BA versus placebo in high CV risk patients reporting clinical outcomes were included.
Main outcomes and measures
Primary efficacy outcomes were major adverse cardiovascular events (MACE), all-cause mortality, CV mortality and non-fatal myocardial infarction (MI). Safety outcomes included new onset or worsening of diabetes mellitus (DM), muscular disorders, gout and worsening of renal function.
Results
Six RCTs with a total of 3956 patients and follow-ups of four to 52 weeks were identified. Heterogeneity mainly derived from differing follow-up duration and baseline CV risk. No difference in MACE (OR 0.84; 95% CI 0.61 to 1.15), all-cause mortality (OR 2.37; CI 0.80 to 6.99) and CV mortality (OR 1.66; CI 0.45 to 6.04) for BA versus placebo was observed. BA showed beneficial trends for non-fatal MI (OR 0.57; CI 0.32 to 1.00) and was associated with a lower risk of new-onset or worsening of DM (OR 0.68; CI 0.49 to 0.94), but higher risk of gout (OR 3.29; CI 1.28 to 8.46) and a trend for muscular disorders (OR 2.60; CI 1.15 to 5.91) and worsening of renal function (OR 4.24; CI 0.98 to 18.39).
Conclusion
BA in high CV risk patients showed no significant effects on major CV outcomes in short-term follow-up. Unfavourable effects on muscular disorders, renal function and gout sound a note of caution. Hence, further studies with longer term follow-up in carefully selected populations are needed to clarify the risk/benefit ratio of this novel therapy.
Keywords: ischaemic heart disease, preventive medicine, clinical pharmacology, clinical trials
Strengths and limitations of this study.
Randomised controlled trials (RCTs) investigating bempedoic acid in patients with high cardiovascular risk and in those with established atherosclerotic cardiovascular disease were included.
Sole inclusion of RCTs may reduce selection bias.
Major clinical outcomes including major adverse cardiovascular events, all-cause mortality, cardiovascular mortality and non-fatal myocardial infarction were analysed.
Low event rates within limited follow-ups may cause imprecise effect estimates.
Heterogeneity in length of follow-up and background lipid-lowering therapy may introduce bias.
Introduction
Hypercholesterolemia is one of the major risk factors of cardiovascular (CV) disease, which is the leading cause of death worldwide.1 The current guideline on the management of blood cholesterol of the American College of Cardiology/American Heart Association recommends to reduce low-density lipoprotein cholesterol (LDL-C) levels by ≥50% in patients at high CV risk, using maximally tolerated statin therapy and—if LDL-C levels remain ≥70 mg/dL—additional non-statin drugs, for example, ezetimibe (class I).2 The European Society of Cardiology 2019 guideline even emphasises a lower LDL-C goal of absolute LDL-C levels <55 mg/dL and a 50% relative LDL-C reduction from baseline in adults at very high CV risk (class I) under intensified lipid-lowering therapy.3 Additional proprotein convertase subtilisin/kexin type 9 (PSCK9)-inhibitors are recommended (class I, for both societies) in patients at very high risk, who are not achieving treatment goals on a maximum tolerated dose of a high-intensity statin and ezetimibe.2–4
Bempedoic acid (BA) is a novel, oral, non-statin, once daily LDL-C lowering drug, which acts as a direct competitive inhibitor of ATP citrate lyase, a key enzyme linking carbohydrate to lipid metabolism with the effect of upregulating hepatic LDL receptor expression and activity.5 Earlier in 2020, both the United States Food and Drug Administration and European Medicines Agency approved BA for treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or established atherosclerotic cardiovascular disease (ASCVD), who require additional reduction of LDL-C despite optimal diet and maximally tolerated statin therapy. Efficacy and safety of additional treatment with BA on maximally tolerated statin therapy have been investigated in randomised controlled trials (RCTs),6–11 however individual trial sample sizes were too small to judge CV efficacy outcomes.
To further evaluate this, we performed a systematic review and meta-analysis of RCTs to investigate BA efficacy with regard to CV outcomes and BA safety—based on all available evidence.
Methods
This systematic review and the accompagnied meta-analysis was performed according to established methods recommended by the Cochrane Collaboration guidelines and the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement.12 13 The review protocol was not registered.
Data sources and search strategy
The online database MEDLINE was systematically searched for published reports up until 1 November 2021. The following keywords were used during searches (in combinations, among others): bempedoic acid, BA, ETC-1002, randomised controlled trial, hypercholesterolemia. Additionally, the Cochrane Central Register of Controlled Trials, Google Scholar, Embase, ClinicalTrials.gov, Clinical Trial Results (www.clinicaltrialresults.org) and the American College of Cardiology web site (www.cardiosource.com) were non-systematically searched for ongoing trials and major congress proceedings. Article bibliographies were additionally screened and relevant articles were added to the systematic review process.
Study selection
All obtained references from primary searches were screened based on title and abstract and categorised further; if content was considered relevant, they were retrieved as full-text reports for detailed evaluation. All controlled trials randomising BA to placebo and reporting CV outcomes, which were available in English language and in full text, were eligible for inclusion. Non-randomised studies were excluded, as were trials without reports of clinical efficacy outcomes and trials investigating PCSK9-inhibitors or inclisiran additionally to BA. No restrictions on follow-up duration, populations or study size were applied.
Efficacy and safety outcomes
Clinical outcomes were defined according to individual study protocols and were analysed as reported. Primary efficacy outcomes of interest were major adverse cardiovascular events (MACE), all-cause mortality, CV mortality and non-fatal myocardial infarction (MI); additional efficacy outcomes of coronary and non-coronary revascularisation, non-fatal stroke, hospitalisation for heart failure and hospitalisation for unstable angina were also analysed. Safety outcomes included new onset or worsening of diabetes mellitus (DM), muscular disorders, gout/elevation in uric acid and worsening of renal function, among others. Drug efficacy on lipid levels was also assessed.
Data collection and quality assessment
Data from included trials were identified, abstracted into prespecified forms and analysed according to the intention-to-treat principle. Cross-checking between investigators was performed to assure internal validity; divergences between investigators were resolved by consensus. Bias risk was appraised13 and the grading of recommendation, assessment, development and evaluation (GRADE) working group certainty rating14 of primary outcomes was performed by two unblinded investigators, who cross-checked each other for errors.
Statistical analyses
RevMan V.5.3 (Cochrane Collaboration) was used for statistical computations. OR and 95% CI were used as summary statistics for dichotomous clinical outcome variables, Forest plots were used for graphical display. The Cochran-Mantel-Haenszel method was applied to compute summary statistics using a fixed-effects model.15 The summary I2 statistic was used to quantify heterogeneity.16–18 Fixed-effects models were used throughout the study due to low I², and a confirmatory analysis using random-effects models19 was additionally performed.
To analyse BA effects on serum lipid levels, data were extracted using mean differences (MD) and SD. SD data in three trials6 7 9 were extracted from published figures using WebPlotDigitizer V.4.2 (https://automeris.io/WebPlotDigitizer/). A fixed-effects model was used to compute summary statistics, again according to the Cochran-Mantel-Haenszel method. Weighted MD with 95% CI were calculated for all lipid level outcome variables. Forest plots were generated for study-specific effect sizes along with 95% CIs and pooled effect measures. An alpha-error probability of p<0.05 was considered statistically significant in all calculations. To ascertain validity of results and account for trial heterogeneity, especially inhomogeneous duration of follow-up, prespecified sensitivity analyses of primary clinical efficacy and safety outcomes stratified by duration of follow-up (short-term (<12 weeks) vs longer term (>12 weeks)) were conducted.
Patient and public involvement
No patient was involved in the study. Furthermore, patients or the public were not involved in the design, conduct, reporting anddissemination plans of our research.
Results
Study selection and patient population
The PRISMA flow chart of the systematic review process is depicted in online supplemental figure 1. Of the 184 studies initially identified, 90 were excluded based on title/abstract and 79 studies for being editorials, reviews, other meta analyses or in vitro studies; nine trials did not meet explicit inclusion criteria due to non-randomised design or non-reporting of clinical outcomes; six studies comprising a total of 3,956 patients were finally included in the meta-analysis.6–11
bmjopen-2021-048893supp001.pdf (10.9MB, pdf)
Study and patients characteristics are reported in tables 1 and 2: five studies were phase 3 RCTs published between 2018 and 2019, Gutierrez et al was a phase 2b RCT published in 2014.11 Three trials included patients treated with a maximally tolerated statin background therapy,7 8 10 three trials with statin intolerance or after after discontinuation of lipid-lowering therapy.6 9 11 Patients were between 55 and 67 years old, most were overweight (average body mass index of 29–31), suffered from a considerable CV risk profile (high rates of ASCVD, DM, HeFH or chronic kidney disease (CKD)) and insufficient control of serum lipid levels (table 2). Duration of follow-up ranged from 4 to 52 weeks.8 10 11
Table 1.
Study characteristics
| Publication, year (acronym) |
Design | Population | Groups | Sample size (n) |
FU (wks) |
Endpoints |
| Ballantyne et al,6 2018 (CLEAR Tranquility) |
RCT (double-blind, phase 3) |
Statin intolerance and LDL-C >100 mg/dL requiring further LDL-C lowering on no more than low-dose statin therapy | BA 180 mg/d+ezetimibe 10 mg/d vs placebo +ezetimibe 10 mg/d |
269 (181 BA; 88 placebo) |
12 | Primary: 12 wk change (%) of LDL-C Secondary: 12 wk change (%) of non-HDL-C, TC, apoB, hs-CRP, TG and HDL-C |
| Ballantyne et al,7 2019 |
RCT (double-blind, phase 3) |
ASCVD and/or HeFH with LDL-C >100 mg/dL, or multiple CVD risk factors with LDL-C >130 mg/dL on maximally tolerated statin therapy | BA 180 mg/d+ezetimibe 10 mg/d vs BA 180 mg/d vs ezetimibe 10 mg/d* vs placebo |
382 (108 BA +ezetimibe; 110 BA; 55 placebo; 109 ezetimibe*) |
12 | Primary: 12 wk change (%) of LDL-C Secondary: 12 wk change (%) of non-HDL-C, TC, apoB, hs-CRP |
| Goldberg et al,8 2019 (CLEAR Wisdom) |
RCT (double-blind, phase 3) |
ASCVD and/or HeFH with LDL-C >70 mg/dL on maximal tolerated lipid-lowering therapy | BA 180 mg/d vs placebo |
779 (522 BA, 257 placebo) |
52 | Primary: 12 wk change (%) of LDL-C Secondary: 24 wk change (%) of LDL-C; 12 wk change (%) of non-HDL-C, TC, apoB and hs-CRP; 12 wk and 24 wk absolute change of LDL-C Tertiary: 52 wk change (%) of LDL-C; 24 wk and 52 wk change (%) of non-HDL-C, TC, apoB, hs-CRP, HDL-C and TG |
| Gutierrez et al,11 2014 |
RCT (double-blind, phase 2b) |
Type 2 diabetes and LDL-C ≥100 mg/dL with a body mass index 25–35 kg/m² without lipid-lowering drugs | BA 80 mg/d for 2 wks followed by 120 mg/d for 2 vs placebo |
60 (30 BA; 30 placebo) |
4 | Primary: 4 wk change (%) of LDL-C Secondary: 4 wk change (%) of TC, non-HDL-C, HDL-C and TG |
| Laufs et al,9 2019 (CLEAR Serenity) |
RCT (double-blind, phase 3) |
Statin intolerance with ASCVD and/or HeFH with LDL-C >100 mg/dL or other patients with LDL-C >130 mg/dL requiring further LDL-C lowering on no more than low-dose statin therapy or other lipid-lowering drugs | BA 180 mg/d vs placebo |
345 (234 BA, 111 placebo) |
24 | Primary: 12 wk change (%) of LDL-C Secondary: 24 wk change (%) of LDL‐C; 12 wk and 24 wk change (%) of non-HDL‐C, TC, apoB, hs-CRP, HDL-C and TG; 12 wk and 24 wk absolute change of LDL-C |
| Ray et al,10 2019 (CLEAR Harmony) |
RCT (double-blind, phase 3) |
ASCVD and/or HeFH with LDL-C >70 mg/dL on maximal tolerated lipid-lowering therapy | BA 180 mg/d vs placebo |
2230 (1488 BA, 742 placebo) |
52 | Primary: number of participants with treatment-related AEs Secondary: 12 wk, 24 wk and 52 wk change (%) of LDL-C, non-HDL-C, TC, apoB and hs-CRP |
Study characteristics of all included trials, regarding study design, study population, characterisation of groups, sample size, follow-up duration and study endpoints.
*Not included in the meta analysis.
AE, adverse events; apoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; BA, bempedoic acid; CVD, cardiovascular disease; d, day; FU, follow up; HDL-C, high-density lipoprotein cholesterol; HeFH, heterozygous familial hypercholesterolemia; hs-CRP, high-sensitivity c-reactive-protein; LDL-C, low-density lipoprotein cholesterol; non-HDL-C, non-high density lipoprotein cholesterol; RCT, randomised controlled trial; TC, total cholesterol; TG, triglycerides; wk, week.
Table 2.
Patients characteristics
| Publication, year | Arms | Age (y) | Female (%) | ASCVD (%) | DM (%) | AHT (%) | BMI (kg/m2) | CKD (%) | TC (mg/dL) | LDL-C (mg/dL) | HDL-C (mg/dL) | Non-HDL-C (mg/dL) | TG (mg/dL) | apoB (mg/dL) | hs-CRP (mg/L) |
| Ballantyne et al6, (CLEAR Tranquillity) | BA | 63.8 | 60.2 | 27.1 | 19.3 | 61.3 | 29.5 | 75.2 | 218.2 | 129.8 | 55.8 | 162.4 | 135.5 | 123.3 | 2.21 |
| Placebo | 63.7 | 63.6 | 25 | 19.3 | 58 | 30.5 | 80.7 | 208.6 | 123 | 57.1 | 151.6 | 153 | 115.8 | 2.26 | |
| Ballantyne et al7 | BA+EZE | 62.2 | 51.2 | 61.6 * | 40.7 | 86 | 31.1 | 65.1 | 237.4 | 153.9 | 49.1 | 188.3 | 156.8 | 121.1 | 3.1 |
| BA | 65 | 54.5 | 62.5 * | 51.1 | 87.5 | 30.6 | 69.3 | 225.5 | 145 | 49.9 | 175.6 | 140.8 | 113.4 | 2.9 | |
| EZE† | 65.1 | 50 | 62.8 * | 50 | 82.6 | 29.9 | 66.3 | 231.3 | 148.9 | 51.4 | 180.2 | 143.5 | 115.5 | 2.8 | |
| Placebo | 65.4 | 41.5 | 63.4 * | 41.5 | 63.4 | 30.7 | 53.6 | 231.3 | 152.8 | 50.3 | 181 | 139.1 | 115.1 | 3 | |
| Goldberg et al8, (CLEAR Wisdom) | BA | 64.1 | 37.2 | 27.1 | 29.7 | 83.9 | 30 | 79.6 | 202.1 | 119.4 | 51.4 | 150.7 | 139.3 | 116.2 | 1.61 |
| Placebo | 64.7 | 34.6 | 25.2 | 31.5 | 87.2 | 30.6 | 78.2 | 204.8 | 122.4 | 51.1 | 153.7 | 143 | 118.6 | 1.88 | |
| Gutierrez et al11 | BA | 55.3 | 43.3 | – | 100 | 26.7 | 30.6 | – | 206.3 | 125.2 | 43.7 | – | 181.5 | – | 2.3 |
| Placebo | 56 | 33.3 | – | 100 | 26.7 | 29.2 | – | 206.7 | 128.4 | 47.4 | – | 152 | – | 2.2 | |
| Laufs et al9 (CLEAR Serenity) | BA | 65.2 | 56.8 | 27.1 | 26.9 | 67.5 | 30.1 | 75.2 | 245.7 | 158.5 | 52.2 | 193.5 | 156.5 | 141 | 2.92 |
| Placebo | 65.1 | 55 | 25.3 | 23.4 | 67.6 | 30.6 | 85.6 | 241.1 | 155.6 | 50.4 | 190.7 | 164 | 141.9 | 2.78 | |
| Ray et al10 (CLEAR Harmony) | BA | 65.8 | 26.1 | 97.4 | 28.6 | 78.9 | – | – | 179.7 | 103.6 | 48.7 | 130.9 | 126 | 88.5 | 1.49 |
| Placebo | 66.8 | 28.7 | 98 | 28.6 | 80.1 | – | – | 178.6 | 102.3 | 49.3 | 129.4 | 123 | 86.8 | 1.51 |
Patient characteristics of all included trials.
*ASCVD and/or heterozygous familial Hypercholesterolemia.
†Not included in the meta analysis.
AHT, arterial hypertension; apoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; BA, bempedoic acid; BMI, body mass index; CKD, chronic kidney disease (estimated glomerular filtration rate <90 mL/min); DM, diabetes mellitus; EZE, ezetimibe; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; non-HDL-C, non-high density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; y, years.
Quality and risk of bias of included studies
All included studies were adequately controlled, double-blind, without incomplete or selective reporting of data indicating a high quality. Some residual risk of bias regarding sequence generation,7 9 11 allocation concealment11 and blinding of outcomes assessor remained unclear.11 Risk of bias assessment of included studies according to Cochrane Collaboration guidelines13 is reported in online supplemental table 1. Certainty rating of consistency of estimated and true effects of primary outcomes according to GRADE working group14 revealed low certainty for MACE and all-cause mortality; certainty for CV mortality and non-fatal MI was rated moderate. GRADE rating is reported in online supplemental table 2.
Bempedoic acid efficacy for cardiovascular outcomes
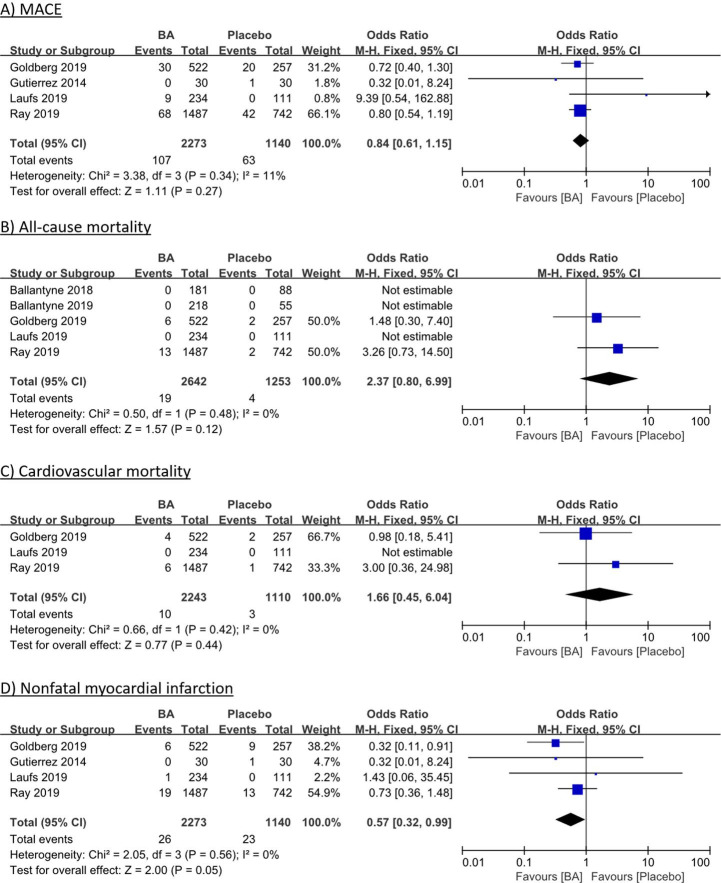
Four RCTs with 3413 patients reported data on MACE (figure 1A),8–11 with no significant difference with BA compared with placebo in meta-analysis (4.7% (BA) vs 5.5% (placebo); OR 0.84, 95% CI 0.61 to 1.15; p=0.27; heterogeneity p=0.34; I2=11%). Five RCTs with 3895 patients were included in the analysis of all-cause mortality and three RCTs with 3353 patients in the analysis of CV mortality (figure 1B, C), but death was a very rare event and occurred only in two studies with longer follow-up.6–10 There was no difference in all-cause mortality (0.7% (BA) vs 0.3% (placebo); OR 2.37; 95% CI 0.80 to 6.99; p=0.12; heterogeneity p=0.48; I2=0%) and in CV mortality (0.4% (BA) vs 0.3% (placebo); OR 1.66; 95% CI 0.45 to 6.04; p=0.44; heterogeneity p=0.42; I2=0%). Data from four RCTs with 3413 subjects were analysed on non-fatal MI (figure 1D),8–11 with a borderline-significant trend towards benefits of BA compared with placebo (1.1% (BA) vs 2.0% (placebo); OR 0.57; 95% CI 0.32 to 0.99; p=0.05; heterogeneity p=0.56; I2=0%).
Figure 1.
Individual and summary ORs with 95% CIs for efficacy outcomes of MACE (A), all-cause mortality (B), cardiovascular mortality (C) and non-fatal myocardial infarction (D) for bempedoic acid vs placebo therapy. Fixed-effects model, Cochran-Mantel-Haenszel estimates; I² measures heterogeneity; BA, bempedoic acid; MACE, major adverse cardiovascular events; M-H, Mantel-Haenszel.
Meta-analysis of additional efficacy outcomes in 3 RCTs with 3353 patients are reported in online supplemental figure 28–10. There were no significant differences in coronary revascularisation (OR 0.82; 95% CI 0.55 to 1.22; p=0.32; online supplemental figure 2A). For non-coronary revascularisation, there was a significant benefit observed in BA versus placebo, although at very low event rates (0.4% (BA) vs 1.1% (placebo); OR 0.41; 95% CI 0.18 to 0.95; p=0.04; heterogeneity p=0.66; I2=0%; online supplemental figure 2B).
There were no significant differences in non-fatal stroke (OR 1.26, 95% CI 0.42 to 3.76; p=0.68; online supplemental figure 2C), hospitalisation for heart failure (OR 2.33; 95% CI 0.67 to 8.11; p=0.19; online supplemental figure 2D) or hospitalisation for unstable angina (OR 0.94; 95% CI 0.51 to 1.74; p=0.84; online supplemental figure 2E).
Bempedoic acid safety outcomes
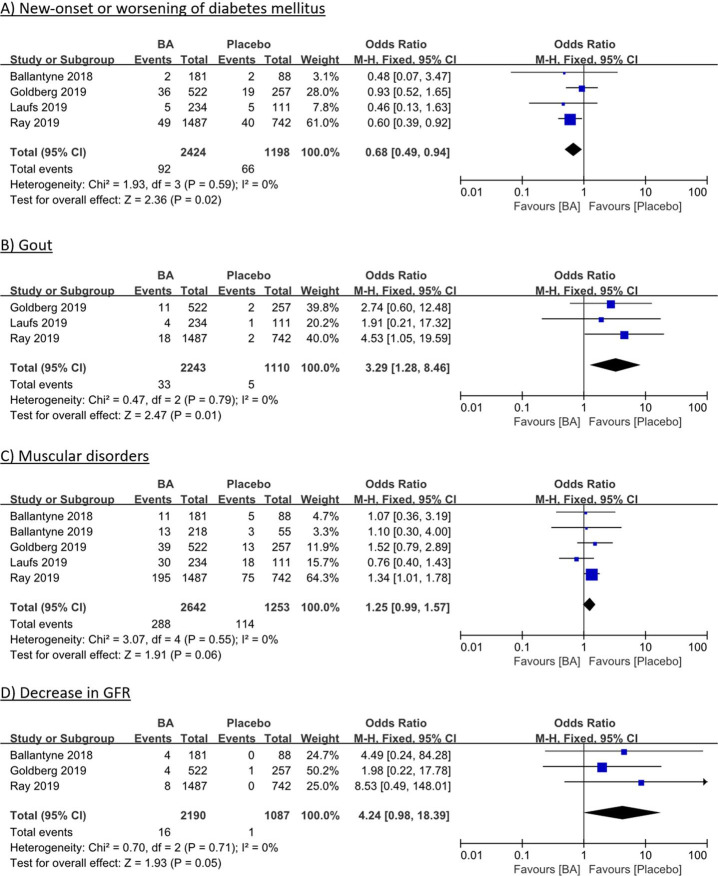
Meta-analysis of four RCTs 6 8–10 comprising 3622 patients showed significantly lower rates of new-onset or worsening of DM for BA versus placebo (3.8% (BA) vs 5.5% (placebo); OR 0.68; 95% CI 0.49 to 0.94; p=0.02; figure 2A). In contrast, however, gout rates were significantly higher in BA treated patients (1.5% (BA) vs 0.5% (placebo); OR 3.29; 95% CI 1.28 to 8.46; p=0.01; figure 2B), which was mediated through elevation of serum uric acid (5.1% (BA) vs 2.0% (placebo); OR 2.60; 95% CI 1.15 to 5.91; p=0.02; online supplemental figure 3A). Muscular disorders were numerically more frequent under BA treatment (10.9% (BA) vs 9.1% (placebo); OR 1.25, 95% CI 0.99 to 1.57; p=0.06; figure 2C). Worsening of renal function was rare but numerically more frequent under BA treatment, evident in decreases of estimated glomerular filtration rate (0.7% (BA) vs 0.1% (placebo); OR 4.24; 95% CI 0.98 to 18.39; p=0.05; figure 2D) and increases in serum creatinine levels (0.8% (BA) vs 0.4% (placebo); OR 2.01; 95% CI 0.67 to 6.02; p=0.21; online supplemental figure 3B).
Figure 2.
Individual and summary ORs with 95% CIs for safety outcomes of new-onset or worsening of diabetes mellitus (A), gout (B), muscular disorders (C) and decrease in GFR (D) for bempedoic vs placebo therapy. Fixed-effects model, Cochran-Mantel-Haenszel estimates; I² measures heterogeneity. BA, bempedoic acid; GFR, glomerular filtration rate; M-H, Mantel-Haenszel.
Additional safety outcomes of upper respiratory tract infection (OR 0.82; 95% CI 0.63 to 1.06; p=0.13; online supplemental figure 3C), urinary tract infection (OR 0.84, 95% CI 0.62 to 1.14; p=0.25; online supplemental figure 3D), neurocongnitive disorders (OR 1.00, 95% CI 0.58 to 1.74; p=0.99; online supplemental figure 3E) and nasopharyngitis (OR 0.88; 95% CI 0.68 to 1.14; p=0.33; online supplemental figure 3F) showed no significant differences between BA and placebo treatment.
Bempedoic acid efficacy for serum lipid levels
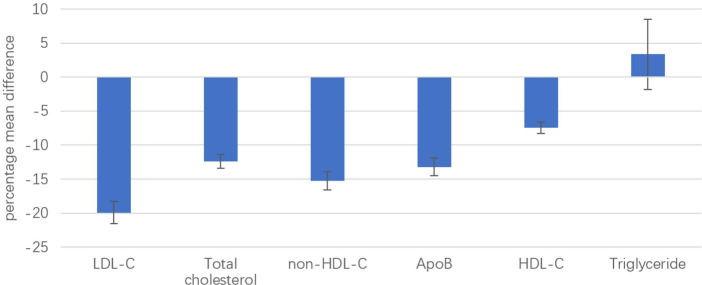
Meta-analysis of effects of BA versus placebo on serum lipid levels is summarised in figure 3, forest plots showing individual and summary MD between groups are presented in online supplemental figure 4. Overall, a MD in LDL-C levels of −19.93% from baseline was observed with the use of BA compared with placebo (95% CI −21.55 to −18.31; p<0.01; online supplemental figure 4A). Treatment with BA also significantly reduced total cholesterol (MD −12.43%; 95% CI −13.42 to −11.43, p<0.01; online supplemental figure 4B), non-high density lipoprotein cholesterol (non-HDL-C) (MD −15.27%; 95% CI −16.59 to −13.95, p<0.01; online supplemental figure 4C) and apolipoprotein B (apoB) (MD −13.20%; 95% CI −14.47 to −11.93, p<0.01; online supplemental figure 4D) compared with placebo. A slight reduction in high-densitiy lipoprotein cholesterol levels was seen under BA compared with placebo (MD −7.5%, 95% CI −8.30 to −6.61, p<0.01; online supplemental figure 4E); BA treatment did not influence triglyceride levels (MD 3.35%, 95% CI −1.78 to 8.49, p=0.20; online supplemental figure 4F).
Figure 3.
Summary mean differences with 95% CIs for BA efficacy on serum lipid levels compared with placebo, for LDL-C, total cholesterol, non-HDL-C, apoB, HDL-C and triglycerides. Fixed-effects model, Cochran-Mantel-Haenszel estimates. apoB, apolipoprotein B; BA, bempedoic acid; HDL-C, high-density-lipoprotein cholesterol; LDL-C, low-density-lipoprotein cholesterol.
Sensitivity analyses
Prespecified sensitivity analyses of primary clinical efficacy and safety outcomes stratified by duration of follow-up (short-term (<12 weeks) vs longer term (>12 weeks)) were conducted to account for heterogeneity of follow-up of included trials. No changes of the overall effects were observed for any of the primary outcomes.
Discussion
This is a systematic review and meta-analysis of all currently available RCT evidence on efficacy and safety of BA versus placebo therapy with respect to clinical outcomes. The main findings are that—compared with placebo—BA therapy had (1) no significant effects on efficacy outcomes of MACE, mortality or MI; (2) significant benefits regarding new-onset or worsening of DM, although detrimental effects on gout and possibly on renal function and muscular disorders; (3) significant decreases of atherogenic serum lipid fractions for example, LDL-C, total cholesterol, non-HDL-C and apoB.
Lowering serum LDL-C to guideline-recommended treatment goals is a cornerstone of CV disease prevention.2 3 Administration of statins is the first-line therapy to reduce serum LDL-C, however a proportion of patients develops statin-associated muscle symptoms and other side effects with impact on treatment adherence.20 21 On the other hand, many patients do not attain treatment goals despite adequate high-intensity statin therapy.22 23 PCSK9-inhibitors—a novel alternative for highest-risk patients—hold disadvantages of high therapy costs and subcutaneous application.24 25 Thus, BA is a promising oral alternative for LDL-C lowering therapy in patients at high CV risk with either statin intolerance or inadequate treatment goal attainment. It has been approved by the United States Food and Drug Administration and European Medicines Agency earlier in 2020.
Several pooled analyses of trials investigating effects of BA have been performed at the same time by other groups.26–30 The majority of those focused on BAs capacities in lipid-lowering with comparable results to the current analysis: Allocation to BA as compared with placebo led to highly significant reductions in major atherogenic lipid fractions of LDL-C, non-HDL-C and apoB.26–29 In contrast, primary interest of the current meta-analysis was to assess evidence on BAs efficacy in improving relevant clinical outcomes, which is the fundamental objective of pharmacological lipid-lowering. The current work is, along with another recent publication,30 the first to provide information on this.
Although BA showed a significant reduction of LDL-C, non-HDL-C and apoB from baseline, current pooled analysis could not find relevant impact on major clinical outcomes. Primarily, duration of follow-up ranging from 4 to 52 weeks across included trials was presumably too short to observe an effect of reduced LDL-C and other atherogenic lipid fractions on major CV outcomes. In addition, combined outcome of MACE associated with higher event rates and than singular outcomes increasing likelihood of detecting benefical treatment effects was extractable from four of six RCTs only, which assumeably may have limited sample size too much to observe short-term effects.
Large-scale RCTs investigating LDL-C lowering agents such as statins, ezetimibe or PCSK9-inhibitors that could demonstrate a beneficial effect of LDL-C lowering on MACE24 25 31 or mortality32 33 in patients with high CV risk had a follow-up that was considerably longer (at least 2.2 to more than 6 years) with larger sample sizes. Benefits of BA on major clinical outcomes could possibly be observed at longer follow-up or if more or larger trials would be retrievable. Additionally, included trials were not conducted exclusively in the setting of secondary prevention, which contributes to heterogeneity of populations regarding baseline CV risk among included studies and requires careful interpretation of results. Whereas in secondary prevention of ASCVD, a pharmacological reduction of LDL-C is known to improve clinical outcomes34—especially at higher baseline LDL-C levels35—evidence of beneficial effects of lowering LDL-C in patients without established ASCVD is less robust.36 However, greatest benefits of lowering LDL-C on CV outcomes and mortality occur in patients with baseline LDL-C levels above 100 mg/dL,35 which lets patient selection in all included trials seem appropriate despite heterogeneous baseline risk and limited transferability of results to other populations. As meta-analysis showed a trend towards reduction of non-fatal MI with BA (OR 0.57; p=0.05) and significantly lower rates of new-onset or worsening of DM with BA (OR 0.68; p=0.02), which is an independent CV risk factor, there are indications that BA possibly holds the potential to improve clinical outcomes in selected patients at high CV risk. A recently published meta-analysis of BAs efficacy for prevention of CV events and diabetes found results differing to the present study. Although only two trials were included in pooled analysis, the authors concluded a significant reduction in MACE. However, studies of Laufs et al and Gutierrez et al were not included for the outcome of MACE despite event rates could be extracted.30
The safety profile of BA found in the current analysis certainly sounds a note of caution that should not be ignored. It has to be questioned, whether adverse effects on muscular disorders (OR 2.60; p=0.03), gout (OR 3.29; p=0.01) and renal function (increase in creatinine OR 3.53; p=0.05), which are also associated with increased CV risk, might counteract BA’s LDL-C lowering potential for CV outcomes.
Further investigation of the risk/benefit ratio of BA in patients at high CV risk is needed to clarifiy the potential role of BA in primary and secondary prevention. Results of the ongoing large-scale CLEAR-Outcomes RCT including approximately 14000 patients (NCT02993406) including high CV risk patients with statin intolerance and baseline LDL-C above 100 mg/dL plans to evaluate an estimated treatment duration of 3.75 years and will help to understand the effects of BA on CV outcomes. Study completion of CLEAR-Outcomes is expected for December 2022.
Limitations
Meta-analysis is currently the only feasible way to explore clinical efficacy and safety of BA, however comes with a number of inherent limitations that arise from analysing secondary or exploratory endpoints in these trials. Low event rates within limited follow-ups cause imprecise effect estimates leading to low-moderate certainty of consistency of estimated and true effects. Variation in length of follow-up may introduce bias; multiple testing bears additional risk. Additional limitations include trial heterogeneity in study comedication (no statin vs maximal tolerated statin, additional ezetimibe) and selection of patients regarding baseline CV risk and potential beneficial effects of lipid-lowering (patients with established ASCVD vs patient at high CV risk). Generally, pooled sample size is still limited compared with other outcome trials in lipid-lowering therapy. Therefore, results of this meta-analysis are exploratory and should be interpreted with caution and evidence is limited to give a recommendation for treatment with BA.
Future directions
If results of large-scale CLEAR-Outcomes RCT (NCT02993406) will be positive for primary endpoint of MACE BA might be an integral part of pharmacological lowering of LDL-C for different reasons. Ambitious treatment goal of LDL-C <55 mg/dL for very high CV risk as given by current European Society of Cardiology guidelines is not achievable in a proportion of patients by ezetimibe added to high-intensity statin only. In many of them, LDL-C is still above treatment goal but <100 mg/dL. In this range, addition of a PCSK9-inhibitor is not assuredly effective in improving outcomes but causes high treatment costs.35 37 Here, BA could be an effective alternative with lower treatment costs when smaller reductions of LDL-C are needed to achieve treatment goal. Moreover, patients with statin-intolerance caused by muscle symptoms not requiring intense LDL-C lowering due to baseline risk or baseline LDL-C might profit from a statin-free regimen including BA and ezetimibe since rates of muscular disorders appear low not markedly exceeding placebo in current meta-analysis. BAs potential in these specific settings has to be evaluated by future adequately designed RCTs analysing relevant clinical outcomes.
Conclusion
Meta-analysis of bempedoic acid versus placebo in patients at high CV risk showed no significant effects on major CV outcomes in short-term follow-up, despite significant reductions of LDL-C and other atherogenic lipid fractions. Unfavourable effects on muscular disorders, renal function and the incidence of gout sound a note of caution. Hence, further studies with longer term follow-up conducted in carefully selected populations are needed to clarify the risk/benefit ratio of this novel therapy.
Supplementary Material
Footnotes
MB and GW contributed equally.
Contributors: YL, MB and GW conceived and designed the study; YL, CP and AK collected sources, selected studies and abstracted data; YL, AK and TK performed doublechecks; YL and GW performed the statistical analysis; all authors analysed and interpreted the data; YL and MB drafted the first manuscript version; NC, AI, MK, MB, CP, VS and GW thoroughly revised it; all authors read, critically revised and accepted the submitted version of the manuscript. GW is responsible for the overall content as guarantor.
Funding: GW was supported by the Forschungskommission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf (No. 2018–32) for a clinician scientist track.
Competing interests: MB reported personal fees for speaking at an expert meeting in lipidology sponsored by Daiichi-Sankyo after primary submission of the current work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study does not involve human participants. An ethics approval is not required for this review and meta-analysis not directly involving humans or animals. Manuscripts of all included individual trials provide an ethics approval statement.
References
- 1.Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes 2019;12:e005375. 10.1161/CIRCOUTCOMES.118.005375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–209. 10.1016/j.jacc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 4.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol 2021:zwab154. 10.1093/eurjpc/zwab154 [DOI] [PubMed] [Google Scholar]
- 5.Feng X, Zhang L, Xu S, et al. Atp-Citrate lyase (ACLY) in lipid metabolism and atherosclerosis: an updated review. Prog Lipid Res 2020;77:101006. 10.1016/j.plipres.2019.101006 [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis 2018;277:195–203. 10.1016/j.atherosclerosis.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2020;27:2047487319864671. 10.1177/2047487319864671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of Bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the clear wisdom randomized clinical trial. JAMA 2019;322:1780–8. 10.1001/jama.2019.16585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of Bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019;8:e011662. 10.1161/JAHA.118.011662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of Bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–32. 10.1056/NEJMoa1803917 [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2014;34:676–83. 10.1161/ATVBAHA.113.302677 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 13.The Cochrane Collaboration . Cochrane Handbook for systematic reviews of interventions. version 6, 2019. Available: https://training.cochrane.org/handbook
- 14.Meader N, King K, Llewellyn A, et al. A checklist designed to aid consistency and reproducibility of grade assessments: development and pilot validation. Syst Rev 2014;3:82. 10.1186/2046-4053-3-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard T, Duffy JC. A Bayesian fixed effects analysis of the Mantel-Haenszel model applied to meta-analysis. Stat Med 2002;21:2295–312. 10.1002/sim.1048 [DOI] [PubMed] [Google Scholar]
- 16.Fleiss JL. Analysis of data from multiclinic trials. Control Clin Trials 1986;7:267–75. 10.1016/0197-2456(86)90034-6 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeger P, Gabrielsson A. Applicability of the Cochran Q test and the F test for statistical analysis of dichotomous data for dependent samples. Psychol Bull 1968;69:269–77. 10.1037/h0025667 [DOI] [PubMed] [Google Scholar]
- 19.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med 2013;158:526–34. 10.7326/0003-4819-158-7-201304020-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen JD, Brinton EA, Ito MK, et al. Understanding statin use in America and gaps in patient education (usage): an Internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15. 10.1016/j.jacl.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 22.Jones PH, Nair R, Thakker KM. Prevalence of dyslipidemia and lipid goal attainment in statin-treated subjects from 3 data sources: a retrospective analysis. J Am Heart Assoc 2012;1:e001800. 10.1161/JAHA.112.001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox KM, Tai M-H, Kostev K, et al. Treatment patterns and low-density lipoprotein cholesterol (LDL-C) goal attainment among patients receiving high- or moderate-intensity statins. Clin Res Cardiol 2018;107:380–8. 10.1007/s00392-017-1193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med Overseas Ed 2017;376:1713–22. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–107. 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 26.Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLoS Med 2020;17:e1003121. 10.1371/journal.pmed.1003121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Ma X, Luo X, et al. Efficacy and safety of bempedoic acid alone or combining with other lipid-lowering therapies in hypercholesterolemic patients: a meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol 2020;21:86. 10.1186/s40360-020-00463-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhagavathula AS, Al Matrooshi NO, Clark CCT, et al. Bempedoic acid and ezetimibe for the treatment of hypercholesterolemia: a systematic review and meta-analysis of randomized phase II/III trials. Clin Drug Investig 2021;41:19–28. 10.1007/s40261-020-00989-1 [DOI] [PubMed] [Google Scholar]
- 29.Banach M, Duell PB, Gotto AM, et al. Association of Bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol 2020;5:1124–35. 10.1001/jamacardio.2020.2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Zhang Y, Tan H, et al. Efficacy and safety of bempedoic acid for prevention of cardiovascular events and diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol 2020;19:128. 10.1186/s12933-020-01101-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 32.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- 33.LIPID Study Group (Long-term Intervention with Pravastatin in Ischaemic Disease) . Long-Term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the lipid trial follow-up. Lancet 2002;359:1379–87. 10.1016/S0140-6736(02)08351-4 [DOI] [PubMed] [Google Scholar]
- 34.Koskinas KC, Siontis GCM, Piccolo R, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J 2018;39:1172–80. 10.1093/eurheartj/ehx566 [DOI] [PubMed] [Google Scholar]
- 35.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018;319:1566–79. 10.1001/jama.2018.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cholesterol Treatment Trialists' (CTT) Collaboration, Fulcher J, O'Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–405. 10.1016/S0140-6736(14)61368-4 [DOI] [PubMed] [Google Scholar]
- 37.Azari S, Rezapour A, Omidi N, et al. Cost-Effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: a systematic review. Heart Fail Rev 2020;25:1077–88. 10.1007/s10741-019-09874-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-048893supp001.pdf (10.9MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data relevant to the study are included in the article or uploaded as supplementary information.