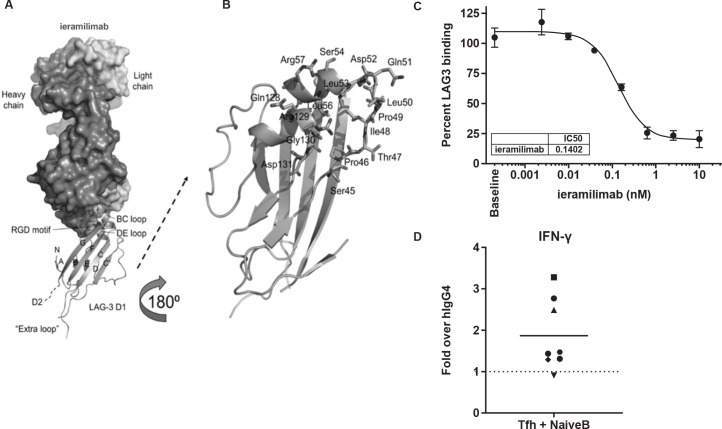
Figure 1.
Preclinical characterization of ieramilimab. (A) Overall structure of ieramilimab antigen-binding fragment binding to LAG-3. Shown are (i) the heavy and light chains of ieramilimab in surface and LAG-3 domain 1 (D1) in ribbons, (ii) the N-terminus (N), the names of the β stands, and the C-terminus of D1 that leads to domain 2 (D2) of LAG-3, (iii) the BC and DE loops of LAG-3 that comprise the epitope of ieramilimab, in which the RGD motif critical for binding MHC-II is shown as spheres, and (iv) the unique ‘extra loop’ of LAG-3, which is far away from the ieramilimab epitope. (B) Detailed view of ieramilimab epitope residues on LAG-3 (shown as sticks and labeled). (C) Ieramilimab blocks the binding of FGL-1 to LAG-3. (D) In three out of eight healthy human donors assayed, ieramilimab enhances IFN-γ secretion in Tfh/B cell co-cultures stimulated with SEB, relative to hIgG4 isotype control. FGL-1, fibrinogen-like protein 1; hIgG4, human immunoglobulin G4; IFN, interferon; LAG-3, lymphocyte-activation gene 3; MHC-II, major histocompatibility complex class II; RGD, arginylglycylaspartic acid; SEB, Staphylococcal enterotoxin B; Tfh, T follicular helper.