Abstract
Background: MOG antibody associated demyelinating disease (MOGAD) is a newly described autoimmune disorder that presents with monophasic or multiphasic demyelination in children. Case: We report a case of MOGAD that was refractory to current treatment algorithms and required rapid escalation of immunotherapy to achieve disease control. Conclusion: This case helps to further expand the phenotype of MOGAD and emphasizes the need to consider MOGAD in patients presenting with focal neurologic deficits, altered mental status, and/or seizures.
Keywords: MOGAD, demyelinating disease, pediatric
Introduction
Myelin oligodendrocyte glycoprotein (MOG) antibody associated demyelinating disease (MOGAD) is a recently described autoimmune disorder that encompasses both monophasic and multiphasic demyelinating syndromes in adults and children. Typical presentations of MOGAD include acute disseminated encephalomyelitis (ADEM), ADEM-ON (optic neuritis), relapsing ON, multiphasic ADEM (MDEM), and neuromyelitis optica spectrum disorder (NMO-SD). Since anti-MOG antibody testing has become clinically available, 18–35% of children who present with acute demyelinating syndromes are reported to be seropositive for anti-MOG antibodies.1–3 A relapsing or multiphasic course is reported in around 30–50% of seropositive patients, and most patients do not require long term immunomodulatory treatment or can achieve disease control with one immunomodulatory agent.3,4 Here, however, we present a case of MOGAD with rapidly relapsing ADEM requiring escalation of immunotherapy beyond the current treatment algorithms.
Case Report
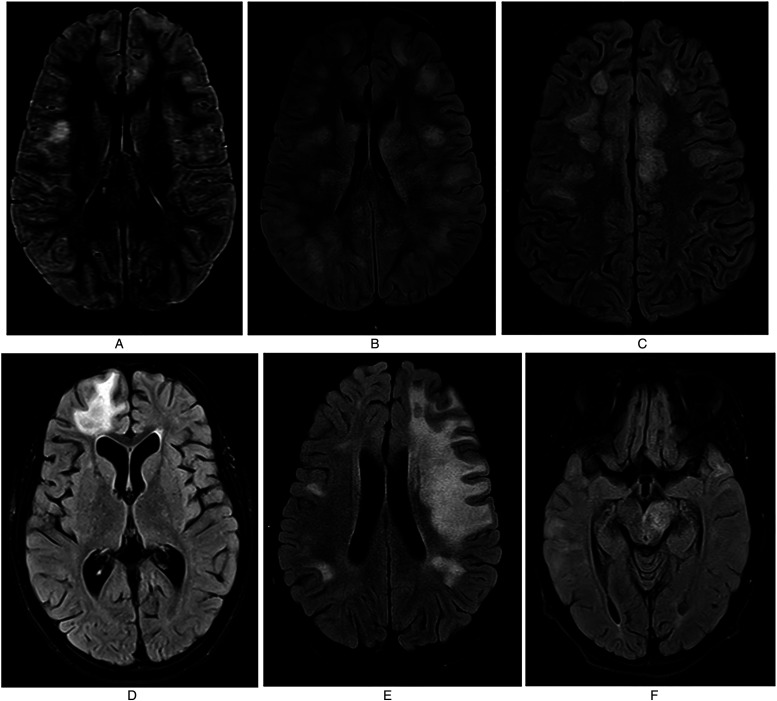
A 10-year-old previously healthy and developmentally appropriate female, with no significant past medical or family history, presented to the emergency room with a 5-day-history of lower extremity weakness, confusion, and fever. Five days prior to presentation, she had tested positive for streptococcal pharyngitis and was treated with benzathine penicillin G by her pediatrician. She then presented to the emergency room due to persistence of fevers and new onset of lower extremity weakness earlier that day. Initial exam was notable for patchy areas of sensory loss, difficulty in standing without assistance due to lower extremity weakness and confusion. Magnetic resonance imaging (MRI) brain was performed and revealed fluffy T2 FLAIR abnormalities consistent with ADEM (Figure 1A). Her MRI spine was normal. She had a lumbar puncture with an unremarkable cell count, but an elevated CSF protein of 74 mg/dl. An extensive infectious work-up was performed, including CSF HSV PCR, enterovirus PCR, Arbovirus PCR, EBV PCR, CMV PCR, HHV6 PCR and cryptococcal antigen, all of which were negative. Broad range bacterial, fungal, and mycobacterial PCRs were also sent on the CSF and were negative. Serum anti-nuclear antibody (ANA) panel, antiphospholipid antibodies, and myeloperoxidase and serine protease 3 antibodies were also checked to evaluate for vasculitic processes, and were unremarkable. She was found to have serum MOG antibodies with a titer of 1:1000. She was admitted to the neurology service and was treated with high dose IV methylprednisolone (IVMP) of 1000 mg daily for 5 days and 2 g/kg of intravenous immunoglobulin (IVIg). The patient quickly returned to her baseline mental status and was discharged home on an oral steroid taper. At discharge, she had only mild residual lower extremity weakness. Four days after discharge from the hospital while she was still on her oral steroid taper, she again began having fevers which were now associated with bilateral eye pain and headaches. She presented to the ER and MRI brain was repeated, which showed improvement in lesion burden. Due to concerns for meningitis, she was treated with broad spectrum antibiotics for 48 hours; however, she became encephalopathic two days into the hospitalization and required transfer to the pediatric ICU. MRI brain was again repeated and showed more extensive T2 FLAIR abnormalities consistent with worsening ADEM and bilateral optic neuritis, though she had normal visual acuity on exam (Figure 1B). She was treated with another seven days of high dose IVMP, 2 g/kg of IVIG over 3 days, and was initiated on rituximab. After this, she returned to her neurologic baseline and a second dose of rituximab was administered two weeks later. She did well for a few weeks after discharge but then again developed daily fevers with headache and eye pain requiring readmission. MRI brain was repeated and showed improvement; however, daily fevers continued, and ten days later she became encephalopathic with generalized fatigue. She again presented to the ER and had an MRI brain that showed new T2 FLAIR lesions (Figure 1C). A B cell panel was obtained to evaluate the efficacy of rituximab, and results were consistent with appropriate B-cell suppression. During this hospitalization, she was again treated with IVMP and 2 g/kg IVIg and quickly returned to her baseline. Due to continued relapses despite treatment with and appropriate response to rituximab, the patient was started on monthly IVIg (1 g/kg) and IVMP (1000 mg) infusions, with plans to alternate so that she received one infusion every two weeks. She did well on this regimen for four months; however, a routine MRI brain was performed which revealed a large tumefactive lesion that spanned the superior and middle frontal gyrus in the left hemisphere (Figure 1D). She was asymptomatic, however given continued breakthrough radiologic disease, she was transitioned to monthly tocilizumab (8 mg/kg) alternating with monthly IVIg (1 g/kg). MRI was obtained after four months on this regimen and showed no new areas of inflammation; thus, tocilizumab was discontinued and only monthly IVIg infusions were continued. Three months later, another MRI brain was obtained and was again stable with no new lesions; however 4 months later, an extensive new lesion was found on routine MRI (patient was asymptomatic), spanning a large portion of the left hemisphere (Figure 1E). Monthly IVIg and tocilizumab infusions were restarted, and an MRI brain performed three months later showed near resolution of the lesion. IVIg was therefore discontinued and she remained on monthly tocilizumab. Shortly after IVIg was stopped, she began having increased drowsiness and confusion, thus MRI brain was repeated and showed extensive new lesions (Figure 1F). Thus, patient was restarted on monthly tocilizumab and IVIg infusions with plans to continue this treatment regimen until she is clinically and radiologically stable for 2 years.
Figure 1.
A: Initial MRI showing bilateral T2 FLAIR hyperintensities throughout concerning for ADEM. Figure 1B: Repeat MRI obtained during second hospitalization (day 26) showing worsening lesion burden with more extensive T2 FLAIR hyperintensities throughout. Figure 1C: New T2 FLAIR lesions seen on MRI during third hospitalization (day 70) after second dose of rituximab. Figure 1D: Repeat MRI obtained during maintenance therapy with alternating IVIg and IVMP therapy on day 160 showing large tumefactive lesion in superior and middle frontal gyrus. Figure 1E: Routine surveillance MRI obtained 1 year after disease onset, after spacing of IVIg therapy, showing hyperintensities spanning a large portion of the left hemisphere. Figure 1F: New T2 FLAIR hyperintensities seen after IVIg was discontinued and patient was maintained solely on monthly tocilizumab monotherapy.
Discussion
MOGAD is a newly described autoimmune disease whose phenotype continues to expand with new patient cases. While initially the existence of positive serum MOG antibodies was thought to be a biological marker for MS, it is now widely agreed that the presence of MOG antibodies typically suggests a non-MS course. 5 The detection of MOG antibodies is most commonly associated with a multitude of demyelinating conditions including ADEM, relapsing optic neuritis (ON), NMOSD, MDEM, and relapsing transverse myelitis (TM); however, MOGAD can also present as autoimmune encephalitis, with altered mental status, seizures, and mainly cortical involvement. Recently, a leukodystrophy-like phenotype has also been described.6,7
Severity of initial presentation, recovery from initial attack and even antibody titers cannot reliably predict which patients will go on to relapse. 8 Because half or more of patients presenting with MOGAD will have a monophasic disease course, the decision of whether or not to initiate chronic immunosuppression at time of initial presentation is a difficult one. At this time, treatment algorithms for MOGAD recommend initiation of immunotherapy only after the patient has a relapse or if there is poor recovery from the first attack.
No clinical trials have been performed in pediatric or adult MOGAD, however typical treatment regimens suggest disease control—in those who require immunotherapy—with use of monthly IVMP, monthly IVIg, mycophenolate mofetil, azathioprine, or rituximab.2,4 There are no reports in the literature of escalation of immunotherapy beyond these treatments due to continued attacks in pediatric patients, which is why this patient case is novel. Tocilizumab use has been reported in adult patients with refractory MOGAD and is used off-label in both adult and pediatric NMO-SD patients.9,10
In conclusion, MOGAD should be on the differential diagnosis in all patients who present with focal neurologic deficits, altered mental status, or seizures with MRI abnormalities consistent with demyelination. Clinicians should recognize that though MOGAD is usually a benign, monophasic condition, in certain patients MOGAD can lead to multiple relapses and ultimately to residual neurologic deficits. Treatment algorithms for MOGAD are available however it is important to recognize that treatment may need to be escalated beyond current algorithms in refractory cases.
Acknowledgments
We would like to acknowledge our patient and family for allowing us to publish this case to help guide treatment for future patients.
Footnotes
Declaration of Conflict of Interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
ORCID iD: Nikita Malani Shukla https://orcid.org/0000-0002-7322-676X
References
- 1.Chitnis T. Pediatric central nervous system demyelinating diseases. Continuum (Minneap Minn. 2019;25(3):793-814. doi: 10.1212/CON.0000000000000730. PMID: 31162317. [DOI] [PubMed] [Google Scholar]
- 2.Marignier R, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762-772. doi: 10.1016/S1474-4422(21)00218-0. PMID: 34418402. [DOI] [PubMed] [Google Scholar]
- 3.Hennes EM, et al. MOG Spectrum disorders and role of MOG-antibodies in clinical practice. Neuropediatrics. 2018;49(1):3-11. doi: 10.1055/s-0037-1604404. Epub 2017 Aug 31. PMID: 28859212. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosius W, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: current insights into the disease pathophysiology, diagnosis and management. Int J Mol Sci. 2020;22(1):100. doi: 10.3390/ijms22010100. PMID: 33374173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziemssen T, Akgün K, Brück W. Molecular biomarkers in multiple sclerosis. J Neuroinflammation. 2019;16(1):272. doi: 10.1186/s12974-019-1674-2. PMID: 31870389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doig D. Autoimmune Cortical Encephalitis in Two Children with Anti-Myelin Oligodendrocyte Glycoprotein (MOG) Antibody in Serum and CSF. 2020. doi: 10.26226/morressier.5e8335ba7cb08a046ef7c7c0. [DOI] [PubMed]
- 7.Hacohen Y, Rossor T, Mankad K, et al. ‘Leukodystrophy-like’ phenotype in children With myelin oligodendrocyte glycoprotein antibody-associated disease. Dev Med Child Neurol. 2017;60(4):417-423. doi: 10.1111/dmcn.13649 [DOI] [PubMed] [Google Scholar]
- 8.Dos Passos GR, Oliveira LM, da Costa BK, et al. MOG-IgG-Associated optic neuritis, encephalitis, and myelitis: lessons learned From neuromyelitis Optica spectrum disorder. Front. Neurol. 2018;9. doi: 10.3389/fneur.2018.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringelstein M, Ayzenberg I, Lindenblatt G, et al. Neuromyelitis Optica study group (NEMOS). interleukin-6 receptor blockade in treatment-refractory MOG-IgG-associated disease and neuromyelitis Optica Spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1100. doi: 10.1212/NXI.0000000000001100. PMID: 34785575; PMCID: PMC859635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breu M, Glatter S, Höftberger Ret al. et al. Two cases of pediatric AQP4-antibody positive neuromyelitis Optica Spectrum disorder successfully treated with tocilizumab. Neuropediatrics. 2019;50(3):193-196. doi: 10.1055/s-0039-1684004. Epub 2019 Mar 26. PMID: 30913570. [DOI] [PubMed] [Google Scholar]