Scheme 3. Synthesis of Compounds 9–12.
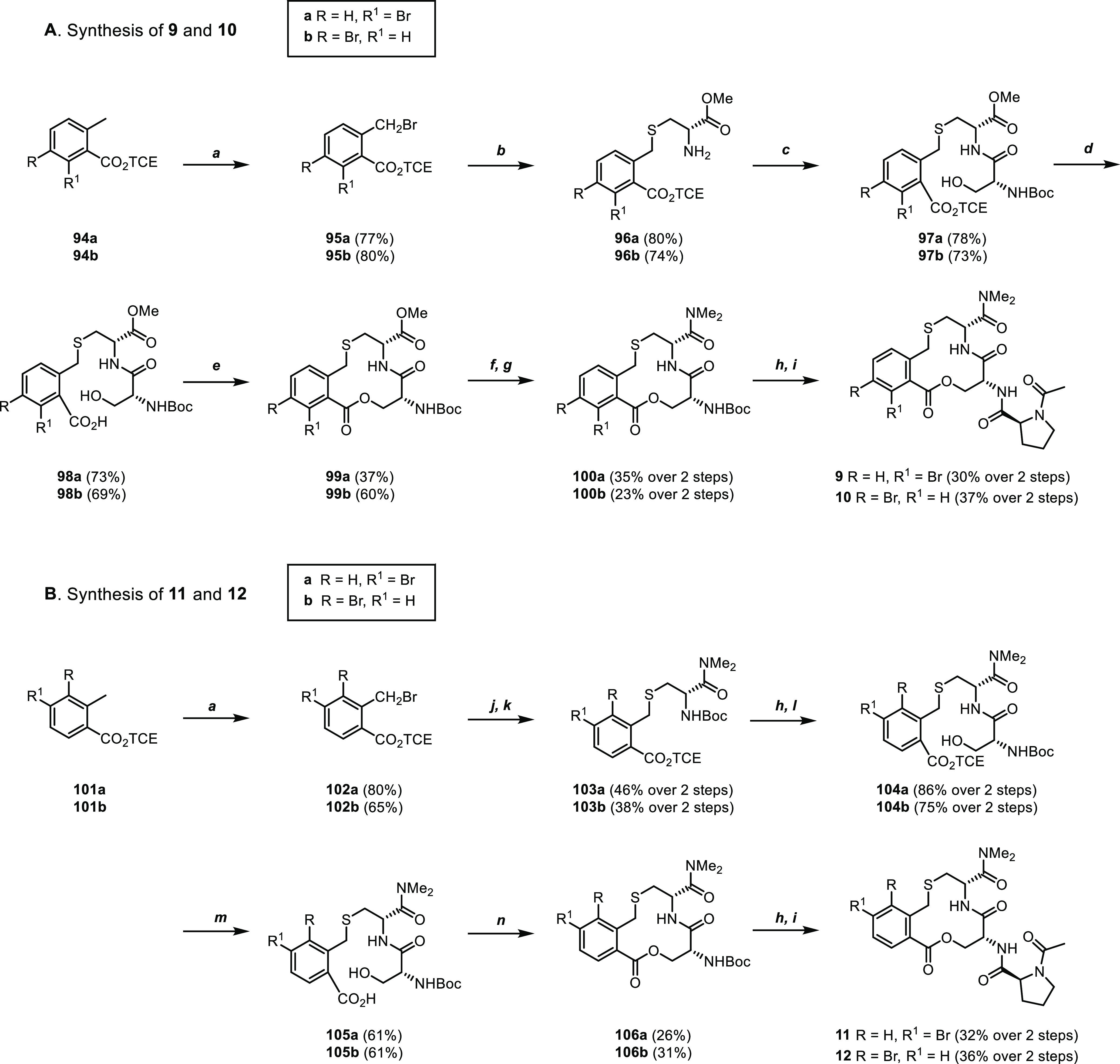
Reagents and conditions: (a) N-bromo succinimide, AIBN, chlorobenzene, 70 °C, 16 h. (b) H-d-Cys-OMe, triethylamine, DMSO, rt, 2 h. (c) Boc-d-Ser-OH, EDC·HCl, MeCN, rt, 1 h. (d) Zn, NH4OAc, THF/H2O 5:1, rt, 2 h. (e) PPh3, DBAD, toluene, rt, 4 h. (f) Me3SnOH, 1,2-DCE, 83 °C, 45 min. (g) 2 M Me2NH in THF, EDC·HCl, HOBt·xH2O, DMF, rt, 2 h. (h) 4 M HCl in 1,4-dioxane, rt, 1 h. (i) Ac-l-Pro-OH, EDC·HCl, DIPEA, DMSO, rt, 2 h. (j) Boc-d-Cys-OH, triethylamine, DMSO, rt, 2 h. (k) Me2NH·HCl, HATU, DIPEA, DMF, rt, 2 h. (l) Boc-d-Ser-OH, EDC·HCl, DIPEA, rt, 1 h. (m) Zn, NH4OAc, THF/H2O 10:1, rt, 2 h. (n) PPh3, DBAD, THF, rt, 4 h.
