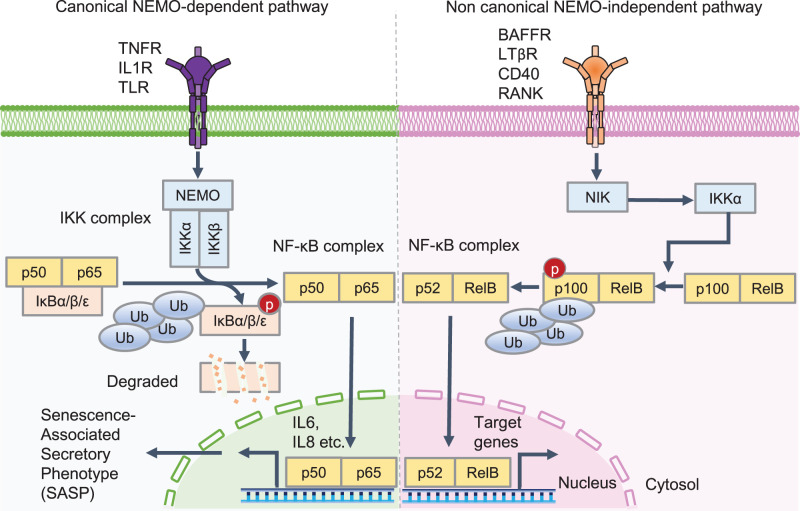
Figure 1. Overview of the canonical and non-canonical NF-κB pathway.
In the canonical nuclear factor-κB (NF-κB) essential modulator (NEMO)-dependent pathway, the inhibitor of κB (IκB) kinase (IKK) complex is activated by activated tumor necrosis factor receptor (TNFR), interleukin (IL)-1 receptor (IL1R), and Toll-like receptor (TLR)s. IKK complex activation induces proteasome-mediated proteolysis of IκB proteins and allows the NF-κB complex (p50/p65) to accumulate in the nucleus. p50/p65 dimers bind to DNA and regulate the transcription of senescence-associated secretory phenotype genes, such as IL-6 and IL-8. In the non-canonical NEMO-independent pathway, NF-κB-inducing kinase (NIK) phosphorylates IKKα and leads to the phosphorylation of p100. This process induces subsequent ubiquitination and partial degradation of p100 by the proteasome to form the NF-κB complex (p52/RelB). p52/RelB dimers enter the nucleus and regulate downstream target genes.