Figure 1. Many proteins can adopt more than more thermodynamically stable microstate with no change in primary structure (sequence), in which the more stable contains an ordered β-sheet ‘amyloid’ structure.
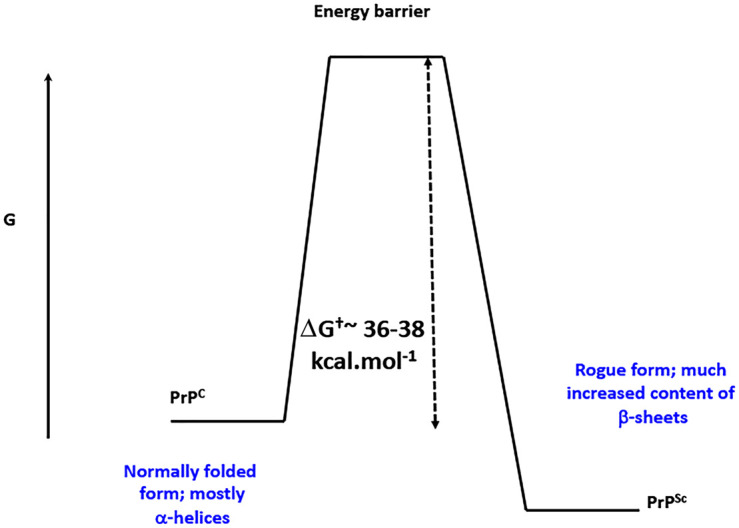
Normally, however, it is present in a less stable state that is kinetically more accessible during and following its synthesis. The more stable (labeled PrPSc) is separated from the initial state (PrPC) via a large energy barrier. This is true for amyloid proteins generally, and is illustrated here for classical prion proteins. Redrawn from a CC-BY publication at [26].
