Abstract
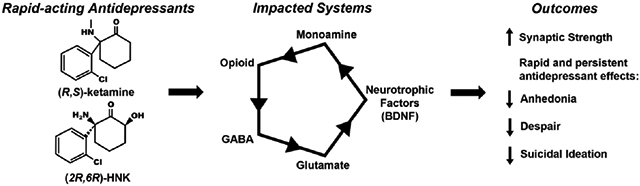
Treating major depression is a medical need that remains unmet by monoaminergic therapeutic strategies that commonly fail to achieve symptom remission. A breakthrough in the treatment of depression was the discovery that the anesthetic (R,S)-ketamine (ketamine), when administered at sub-anesthetic doses, elicits rapid (sometimes within hours) antidepressant effects in humans that are otherwise resistant to monoaminergic-acting therapies. While this finding was revolutionary and led to the FDA approval of (S)-ketamine (esketamine) for use in adults with treatment-resistant depression and suicidal ideation, the mechanisms underlying how ketamine or esketamine elicit their effects are still under active investigation. An emerging view is that metabolism of ketamine may be a crucial step in its mechanism of action, as several metabolites of ketamine have neuroactive effects of their own and may be leveraged as therapeutics. For example, (2R,6R)-hydroxynorketamine (HNK), is readily observed in humans following ketamine treatment and has shown therapeutic potential in preclinical tests of antidepressant efficacy and synaptic potentiation while being devoid of the negative adverse effects of ketamine, including its dissociative properties and abuse potential. We discuss preclinical and clinical studies pertaining to how ketamine and its metabolites produce antidepressant effects. Specifically, we explore effects on glutamate neurotransmission through N-methyl D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), synaptic structural changes via brain derived neurotrophic factor (BDNF) signaling, interactions with opioid receptors, and the enhancement of serotonin, norepinephrine, and dopamine signaling. Strategic targeting of these mechanisms may result in novel rapid-acting antidepressants with fewer undesirable side effects compared to ketamine.
Keywords: Ketamine; (2R,6R)-Hydroxynorketamine; Antidepressant; Glutamate; Depression; NMDAR
Graphical Abstract
1. Introduction
In 2019, the World Health Organization (WHO) estimated that depression was the second most burdensome mental health disorder, behind substance abuse, in the United States as measured by disability-adjusted life years (DALYs) i.e., the number of healthy years of life lost to disability (Geneva, 2020). Depression is a risk factor for obesity, cardiovascular disease, type 2 diabetes, substance abuse, and cancer (Currier & Nemeroff, 2014; Dhar & Barton, 2016; Holt et al., 2014; Kessler, 2004; Ouakinin et al., 2018). Additionally, patients with depression are at an increased risk of suicide (Conwell et al., 1996; Henriksson et al., 1993). It is estimated that the economic cost of depression in the United States is immense, costing hundreds of billions of dollars annually (Greenberg et al., 2021). Thus, there is tremendous economic and social incentive to find novel therapeutics for depression, as it could alleviate significant non-communicable disease burden and loss of life. Unfortunately, current therapeutic tools have failed to reduce the prevalence of depression, with many patients not responding to, and most having significant side effects from, first-line treatments such as selective serotonin reuptake inhibitors (SSRIs) (Locher et al., 2017; Trivedi et al., 2006). Additionally, SSRIs and other traditional antidepressants are problematic as they typically take weeks before therapeutic effects are observed and require daily administration (Entsuah et al., 2001; Thase et al., 2005). Thus, a paradigm shift in therapeutic strategy was needed for depression, with an emphasis on rapid relief of symptoms.
A major breakthrough in the treatment for depression was the observation that racemic (R,S)-ketamine (henceforth referred to as ketamine), well characterized pharmacologically as an NMDA receptor (NMDAR) antagonist, produced rapid and long-lasting antidepressant effects in humans (Daly et al., 2018; Zarate, Singh, Carlson, et al., 2006). Ketamine was first derived from phencyclidine (PCP) and administered to humans in the 1960s with the goal of establishing novel anesthetic compounds. Indeed, ketamine produced a dissociative, anesthetized state in humans which led to its use as an anesthetic and analgesic (Domino, 2010). However, the antidepressant effects of ketamine were not fully realized until decades later. The clinical use of NMDAR antagonists, such as ketamine, for the treatment of depression was preceded by the finding that other NMDAR antagonists, including AP-7 and MK-801, produced antidepressant-like effects in mice (Trullas & Skolnick, 1990). A decade later, a seminal study of ketamine for use in adults with depression observed a significant reduction in depression symptoms as measured by the Hamilton Depression Rating Scale (HDRS) within four hours post-ketamine treatment (0.5 mg/kg intravenously [i.v.] over 40 minutes) relative to placebo (Berman et al., 2000). The second study observed a reduction in depression symptoms in treatment-resistant patients (who had failed an average of six antidepressant trials) 110 minutes following ketamine treatment (0.5 mg/kg i.v. over 40 minutes) relative to placebo as measured by the HDRS. Remarkably, the effects of the single ketamine treatment persisted in some patients for a week or longer (Zarate et al., 2006). To improve the ease of drug delivery, an (S)-ketamine nasal spray was developed and assessed for antidepressant efficacy in patients with treatment-resistant depression (Daly et al., 2018). This study observed a similar rapid reduction (within two hours) in depression symptoms as measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) following an intranasal (S)-ketamine treatment (28 or 84 mg), which separated from placebo even at one week following a single administration. A follow-up report observed that prolonged treatment (weekly or biweekly treatment) with (S)-ketamine nasal spray (56-84 mg; 16 weeks), in conjunction with an oral antidepressant (SSRI or a selective norepinephrine reuptake inhibitor [SNRI]), was well-tolerated with significant remission of symptoms. Further, patients who were in remission were more likely to relapse when switched to a placebo nasal spray in conjunction with an oral antidepressant (Daly et al., 2019). Considering the positive outcomes of these clinical studies, (S)-ketamine appears to alleviate significant symptom burden for depression which led to the (S)-ketamine nasal spray (under the brand name Spravato®) receiving FDA approval in March of 2019 as an adjunct treatment for adults with treatment-resistant depression, or major depression with suicidal thoughts or actions (Cristea & Naudet, 2019). However, this approval and the use of ketamine generally to treat depression has met some criticism concerning the adverse effects associated with (S)-ketamine treatment, especially the dissociative properties, as well as the potential for abuse that limit patients’ treatments to specialized clinics or hospital settings (Gastaldon et al., 2019; Liu et al., 2016; Turner, 2019). In response, these limitations have increased research in the cellular and molecular mechanisms that facilitate the antidepressant and adverse effects of ketamine and has invigorated efforts into researching other novel rapid-acting antidepressants that act on the NMDAR and psychedelic compounds such as psilocybin (Carhart-Harris et al., 2021; Davis et al., 2021; Gould et al., 2019).
An emerging viewpoint is that the mechanism of action of ketamine may be related to the pharmacological properties of its metabolites. While the metabolism of ketamine has been thoroughly reviewed and illustrated elsewhere, ketamine is rapidly metabolized resulting in neuroactive products that are likely to contribute to its therapeutic effects (Highland et al., 2021; Zanos et al., 2018). Ketamine undergoes its first metabolic transformation into (R,S)-norketamine in the liver (Figure 1). Next, (R,S)-norketamine can either be converted into (R,S)-dehydronorketamine (DHNK) or into (R,S)-hydroxynorketamine (HNK). Notably, there are 12 HNKs that have been detected in human plasma following ketamine treatment which are categorized based on the positioning of a hydroxyl group on the cyclohexyl ring (in position 4, 5, or 6) and their stereochemistry at two stereocenters (R,R; S,S; R,S; or S,R). Norketamine, DHNK, and (2R,6R;2S,6S)-HNK in particular are detected in human plasma as early as 40 minutes following an antidepressant dose of ketamine (0.5 mg/kg i.v. over 40 minutes) with the most abundant HNKs being (2R,6R;2S,6S)-HNK and (2S,6R;2R,6S)-HNK (Moaddel et al., 2010; Zarate et al., 2012; Zhao et al., 2012). Importantly, ketamine, norketamine, and (2R,6R)-HNK are all detected in brain tissue samples of mice within 10 minutes post-ketamine administration (at 10 mg/kg, i.p.) which suggests that these three compounds have the potential to produce rapid therapeutic or adverse effects that have yet to be fully characterized (Zanos et al., 2016). DHNK is not detected in brain tissue samples in mice following ketamine administration, and presumably not in humans either (Can et al., 2016). Based on studies that detail the onset of drug-induced effects in humans, ketamine (0.5 mg/kg i.v. over 40 minutes) produces illusory experiences, increases in perceptual acuity, and paranoia within 10 minutes of treatment initiation, which supports rapid brain entry of ketamine in humans (Krystal et al., 1994). Regarding elimination of ketamine and its metabolites, the elimination half-life is between two to four hours, varying slightly with the route of administration (Clements et al., 1982). Plasma levels of ketamine, norketamine, DHNK, and (2R,6R)-HNK were still detectable (>4 ng/mL) one-day post-ketamine infusion (0.5 mg/kg i.v. over 40 minutes) in patients (Zarate et al., 2012). Collectively, these observations highlight that antidepressant doses of ketamine readily enter the brain and are rapidly metabolized.
Figure 1: Ketamine metabolism.
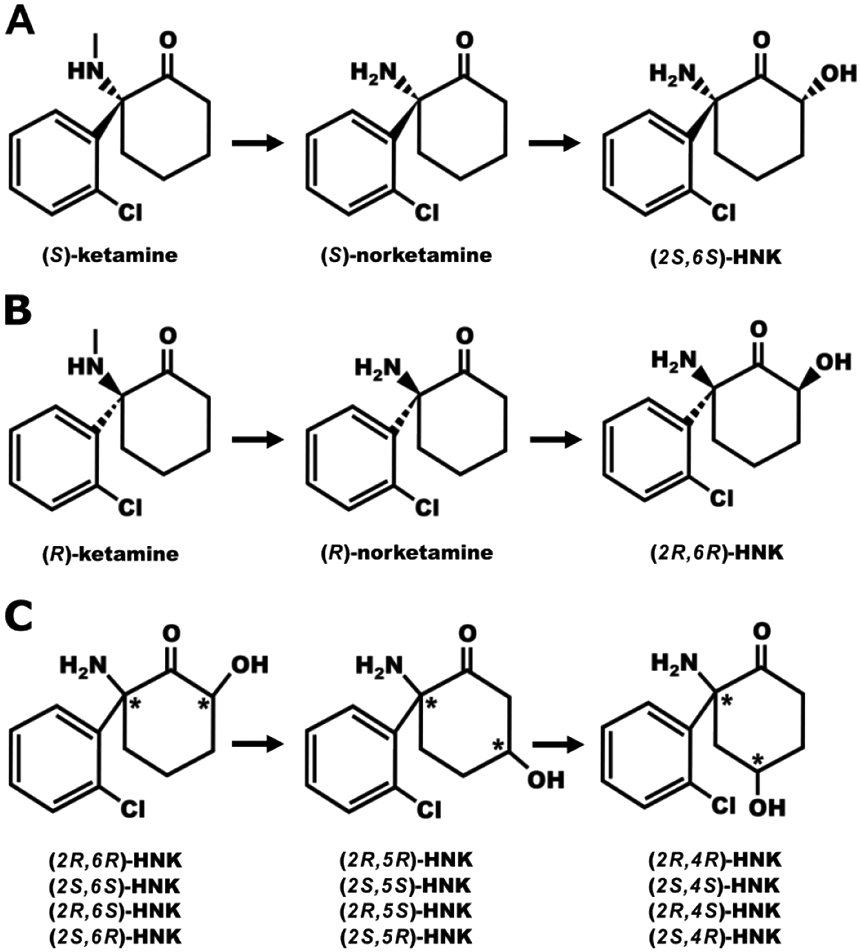
A, B) The (S) and (R) enantiomers of ketamine are rapidly and stereoselectively metabolized by liver P450 enzymes to produce their respective norketamine enantiomer via demethylation of the methyl amine and then hydroxylation of the cyclohexanone ring to produce the hydroxynorketamines (HNKs). The production of dehydroxynorketamine from norketamine is not shown as the dehydroxynorketamines do not appear to cross the blood brain barrier (Can et al., 2016). C) The HNKs (12 in total) are named based on the positioning of hydroxyl group on the cyclohexanone ring and stereochemistry of the hydroxyl and amino groups. For example, (2R,6S)-HNK denotes on the cyclohexanone ring the amino group positioned at carbon 2 in the R configuration and a hydroxyl group at carbon 6 in the S configuration. *Denotes location of a stereocenter.
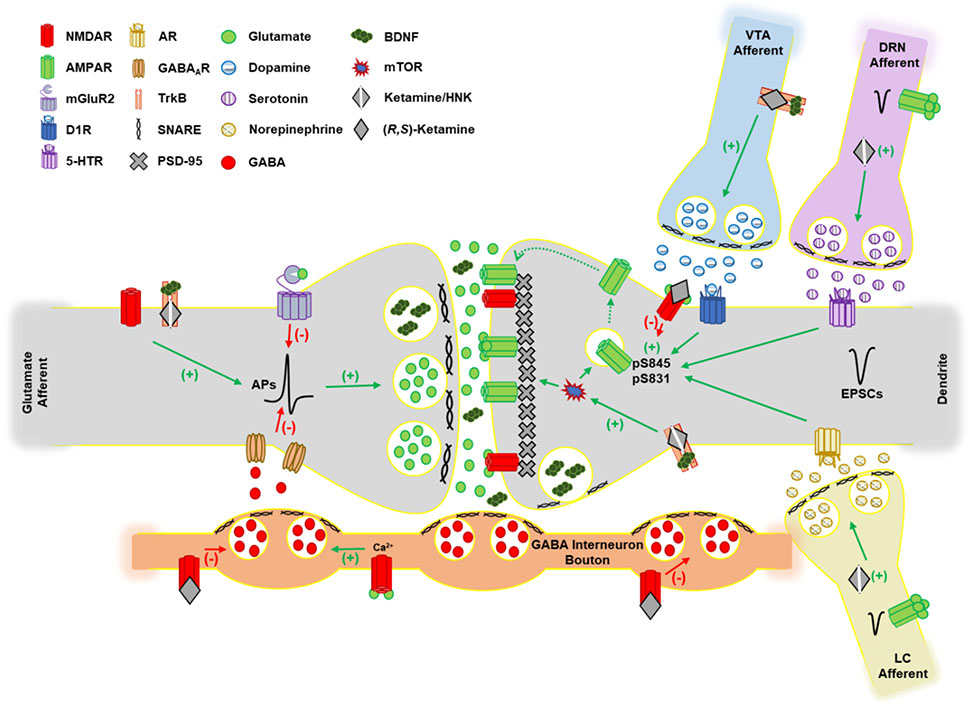
The mechanism of action of ketamine and its metabolites as antidepressants remain to be fully resolved. Ketamine was originally characterized as an open-channel blocker of the N-methyl D-aspartate receptor (NMDAR), an ionotropic glutamate receptor, and there is evidence that NMDAR antagonism is responsible for ketamine’s dissociative and psychotomimetic effects (Krystal et al., 1994; Zorumski et al., 2016). These properties are mimicked by other open-channel blockers such as PCP and MK-801 which are commonly used to model schizophrenia in preclinical studies (Jentsch & Roth, 1999). Given that the evidence of PCP, MK-801, and memantine exerting antidepressant-like effects in rodents is mixed, with clinical studies either failing or lacking, NMDAR antagonism may not fully explain the antidepressant effects of ketamine (Autry et al., 2011; Gould et al., 2019; Hillhouse & Porter, 2014; Hillhouse et al., 2014; Trullas & Skolnick, 1990; Zanos et al., 2016). In support, memantine, which blocks the NMDAR ion channel at the same site as ketamine, failed to exert antidepressant effects in patients with depression (Omranifard et al., 2014; Smith et al., 2013; Zarate, Singh, Quiroz, et al., 2006). However, another NMDAR channel blocker AZD6765 (lanicemine, 150 mg i.v.), transiently improved MADRS scores at 80- and 110-minutes post-treatment in patients with major depression that were otherwise unmedicated (Zarate et al., 2013). Another study also observed that lanicemine (100 mg i.v.) when administered every other day for three weeks as an adjunctive treatment improved MADRS scores in patients with major depression starting at the second week of treatment through two weeks post-treatment (Sanacora et al., 2014). Notably, this study did not observe rapid antidepressant effects. A large phase III study that utilized a 12-week treatment course totaling 15 treatments in patients with major depression failed to observe an improvement in MADRS scores relative to placebo (Sanacora et al., 2017). Given these observations, NMDAR antagonism is unlikely to be the sole mechanism of action that produces antidepressant effects. Indeed, an emerging conclusion is that ketamine and its metabolites may have effects that are both dependent and independent of NMDAR antagonism, impacting several neurotransmitter systems to elicit their antidepressant effects (Figure 1). The following sections describe how different neurotransmitter systems and signaling pathways may contribute to the antidepressant effects of ketamine and its metabolites.
2. Glutamate Signaling
The ability for ketamine to elicit rapid behavioral effects may be due to its profound impact on glutamatergic signaling – the primary excitatory neurotransmitter system in the brain. Ketamine was initially characterized as an NMDAR antagonist (Anis et al., 1983; Zorumski et al., 2016). NMDARs are heterotetrameric ligand-gated ionotropic receptors (Hansen et al., 2021; Traynelis et al., 2010). There are three principal subunit types for NMDARs: GluN1, GluN2, and GluN3 with GluN1 being obligatory for cell surface expression. GluN1 has 8 known subtypes generated from alternative splicing whereas GluN2 and GluN3 have subtypes generated from different genes. GluN2 has 4 subtypes designated as A, B, C and D whereas GluN3 has an A and B subtype. Typically, the quaternary structure of NMDARs is comprised of two GluN1 and two GluN2 subunits. The GluN3 subunit is developmentally regulated and more scarcely expressed in the adult brain and is thought to substitute for a GluN2 subunit to form GluN1-GluN2-GluN3 tri-heterotetrameric receptor. The NMDAR has two ligand binding sites, with GluN2 binding to the primary agonist glutamate whereas GluN1 and GluN3 bind glycine or D-serine, in which binding is obligatory for activation by glutamate (Paoletti & Neyton, 2007; Pérez-Otaño et al., 2016). Agonist binding alone is insufficient for ion conductance through NMDARs, as the ion channel pore is often blocked at resting membrane potential by a Mg2+ ion. To overcome Mg2+ block, antecedent membrane depolarization is required. This often occurs through activation of the ionotropic glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) which are also heterotetramers comprised of a combination of GluA1,2,3, and 4 subunits which rapidly conduct Na+, K+, and sometimes Ca2+ depending on their subunit composition (Henley & Wilkinson, 2016). With Mg2+ lifted, the NMDAR is permeable to Na+, K+, and importantly, Ca2+ which serves as a critical intracellular signal that regulates a variety of second messenger systems that influence cellular physiology and information transfer. Notably, the magnitude of Mg2+ block and Ca2+ permeability is dependent on the subunit composition of the receptor, with GluN2A/B-containing NMDARs being more prone to Mg2+ block over GluN2C/D and GluN3A/B being less permeable to Ca2+ (Kuner & Schoepfer, 1996; Matsuda et al., 2003; Monyer et al., 1992; Perez-Otano et al., 2001). The significance of these varying subunit combinations is that they confer unique biophysical characteristics to the NMDAR, which tunes the information conveyed by their activation and thus has important implications for the pharmacodynamics of antagonists such as ketamine.
Ketamine impacts the NMDAR via an open-channel block mechanism which makes it a non-competitive antagonist. Much like Mg2+, ketamine is trapped within the ion pore of activated receptors to prevent ion conductance and remains bound upon channel closing and can dissociate at depolarized membrane potentials upon channel re-opening (MacDonald et al., 1987; MacDonald & Nowak, 1990; Y. Zhang et al., 2021; Zorumski et al., 2016). Importantly, the magnitude of ketamine block appears to be modulated by many factors, including membrane potential, subunit composition, extracellular pH, and the presence of obligatory agonists (Table 1). For instance, GluN2C/D-containing NMDARs expressed in HEK293T cells appear to be more susceptible to ketamine block (IC50 of 1.18 and 2.95 μM, respectively) in the presence of physiological 1 mM Mg2+, 1 mM glutamate, 100 μM glycine, and pH 7.2 relative to GluN2A/B (5.35 and 5.08 μM, respectively) containing NMDARs (Kotermanski & Johnson, 2009). Importantly, in the absence of Mg2+, the IC50 values of ketamine are lower for GluN2A/B/C/D (0.33, 0.31, 0.51, and 0.83 μM respectively). These authors suggest that ketamine may have some selectivity for GluN2C/D-containing NMDARs in the presence of physiological Mg2+ as these subunits are naturally less prone to Mg2+ block and Mg2+ competes with ketamine for binding to GluN2A/B-containing NMDARs. On the other hand, another study showed that the IC50 values of ketamine at GluN2A/B/C/D-containing NMDARs expressed in Xenopus laevis oocytes in the presence of 50 μM glutamate, 30 μM glycine, and in the absence of Mg2+ at a pH of 7.6, are 3.3, 0.9, 1.7, and 2.4 μM, respectively, which suggests that GluN2B is most vulnerable to ketamine antagonism (Dravid et al., 2007). Additionally, lowering pH from 7.6 to 6.9 reduced the IC50 values of ketamine at all NMDAR subunits (2A, 0.537; 2B, 0.239; 2C, 0.66; 2D, 1.23 μM). The discrepancy in ketamine IC50 values between these two studies might be explained by the concentration of agonist used (50 μM versus 1 mM glutamate), as higher agonist concentrations allow non-competitive antagonists, e.g. ketamine, to block more effectively due to a greater number of open channels (Lipton, 2006). Notably, 50 μM and 1 mM glutamate roughly estimate extrasynaptic and active synaptic glutamate levels, respectively (Dzubay & Jahr, 1999; Moussawi et al., 2011). Likewise, the pH used by Kotermanski and Johnson (2009) was 7.2, which falls within the pH range of 7.6 and 6.9 used by Dravid et al. (2007), and may explain why the IC50 values differ between these studies. Regarding native NMDARs in hippocampal neurons, the IC50 value for ketamine is 0.43 μM in the absence of Mg2+ and at a pH of 7.4. The IC50 observed in striatal neurons is higher than at hippocampal neurons at 0.92 μM, which suggests that the potency of ketamine to inhibit NMDARs may differ across individual brain regions (Parsons et al., 1996). Given these findings, it is likely that the effects of ketamine on NMDAR function are influenced by the presence of Mg2+, agonist concentration, subunit composition, and pH, all of which may vary as a function of brain region. These variables are critical to consider when translating in vitro findings into ex vivo or in vivo systems, as these variables are likely to vary within the local microenvironment of the synaptic cleft to impact ketamine pharmacodynamics. However, how the antidepressant effects of ketamine are influenced by these variables remains to be fully examined.
Table 1: Inhibitory potency of ketamine, (2S,6S)-HNK, and (2R,6R)-HNK of the NMDAR.
Top: half-maximal inhibitory concentration (IC50) at recombinant NMDARs expressed in Xenopus oocytes that contain rat 2A, 2B, 2C, or 2D subunits co-expressed rat GluN1. Bottom: IC50 to inhibit field excitatory post-synaptic potentials produced by NMDARs in the CA1 region of mouse hippocampal slices. Rank order of potency is (R,S)-ketamine > (2S,6S)-HNK > (2R,6R)-HNK. Additional studies have investigated the interaction between ketamine, its metabolites, and the NMDAR (Abbott & Popescu, 2020; Highland et al., 2021; Moaddel et al., 2013; Morris et al., 2017; Zanos et al., 2016).
| Drug | Dependent Variable | IC50 (μM) | References |
|---|---|---|---|
| (R,S)-ketamine | GluN1/2A current amplitude | 0.33-5.35 | (Dravid et al., 2007; Kotermanski & Johnson, 2009) |
| GluN1/2B current amplitude | 0.239-5.08 | ||
| GluN1/2C current amplitude | 0.51-1.70 | ||
| GluN1/2D current amplitude | 0.83-2.95 | ||
| CA1 NMDAR-dependent fEPSP slope | 4.5 | (Lumsden et al., 2019) | |
| (2R,6R)-HNK | GluN1/2A current amplitude | 498 | (Lumsden et al., 2019) |
| GluN1/2B current amplitude | 258 | ||
| GluN1/2C current amplitude | 202 | ||
| GluN1/2D current amplitude | 287 | ||
| CA1 NMDAR-dependent fEPSP slope | 211.9 | ||
| (2S,6S)-HNK | GluN1/2A current amplitude | 43 | (Lumsden et al., 2019) |
| GluN1/2B current amplitude | 21 | ||
| GluN1/2C current amplitude | 15 | ||
| GluN1/2D current amplitude | 13 | ||
| CA1 NMDAR-dependent fEPSP slope | 47.2 |
One of the effects of ketamine administration in rodents is a facilitation of AMPAR-mediated signaling and the strength of glutamatergic synapses, which is associated with antidepressant-like effects and as hypothesized in a 2006 review by Alt and colleagues, may represent a general mechanism through which relief of symptoms is achieved (Alt et al., 2006). In support, a seminal study observed that the rapid (within 30 minutes) antidepressant-like effects, as measured by immobility time in the forced swim test, of ketamine (2.5 mg/kg i.p.) and the GluN2B specific antagonist Ro25-6981 (3 mg/kg i.p.) in mice are blocked by the AMPAR antagonist NBQX (Maeng et al., 2008). Notably, this study also found that the effects of Ro25-6981 were not sustained for as long as ketamine (two weeks post-treatment) which suggests that NMDARs may not be the only locus for the mechanism of action of the long-term actions of ketamine. Another study in the rat prefrontal cortex (PFC) observed that ketamine (10 mg/kg i.p.) induces the phosphorylation of the key intracellular messengers 4E-PB1, p70S6K, mTOR, ERK, and Akt one hour post-treatment (Li et al., 2010). This phenomenon corresponded to an increase in the key synaptic proteins ARC, synapsin 1, PSD95, and GluA1 (the obligatory AMPAR subunit) two hours post-treatment and an increase in excitatory postsynaptic current (EPSC) amplitude at thalamocortical and intracortical synapses 24 hours post-treatment. Intriguingly, Ro25-6981 (10 mg/kg i.p.) mimicked the effects of ketamine on mTOR signaling components one hour post-treatment and synaptic proteins (synapsin1, PSD95, and GluA1) six hours post-treatment. Notably, another study in mice found that the mTOR effectors 4E-BP1 and 4E-BP2 are necessary for the antidepressant-like effects of ketamine (10 mg/kg i.p.) and (2R,6R)-HNK (20 mg/kg i.p.) in the forced swim and novelty suppressed feeding tests when administered one hour prior to testing (Aguilar-Valles et al., 2021). The effects of ketamine (20 μM, 30-minute bath application) in this study were correlated to an enhancement in excitatory signaling in CA1 slices. Furthermore, others have established that ketamine (20 μM, 30-minute bath application) potentiates AMPAR-mediated evoked neurotransmission in the CA1 of mice independent of changes in presynaptic release probability (Autry et al., 2011; Nosyreva et al., 2013). Likewise, (2R,6R)-HNK (10 μM, 60-minute bath application) was observed to increase AMPAR-mediated synaptic strength in the CA1 of rats, however it appears that this effect is likely presynaptic due to an increase in glutamate release probability (Riggs et al., 2020). At the molecular level, ketamine increases phosphorylation of GluA1 at serine residue 845 (S845). When phosphorylated, this site selectively increases extrasynaptic insertion of AMPARs which can then diffuse to the synapse to promote long-term potentiation (LTP) (Derkach et al., 2007; Oh et al., 2006; Sun et al., 2005). A study found that ketamine (20 μM bath application) enhances phosphorylation of GluA1 S845 at the SC-CA1 synapse of mice up to two hours post-treatment and its rapid (30 minutes) and long-term (7 days) antidepressant-like effects in the forced swim test (10 mg/kg i.p.) are blocked in mice with S845 mutated to an alanine residue that cannot be phosphorylated (Zhang et al., 2016). Likewise, another study in rats found that ketamine (20 μM bath application) increased GluA1 expression and S845 phosphorylation 30 minutes post-treatment and was maintained for 4 hours (Zhang et al., 2017). Notably, phosphorylation of GluA1 at S845 is necessary but not sufficient for synaptic incorporation (Esteban et al., 2003). Further, NMDAR activation is inversely correlated with GluA1 S845 phosphorylation, with NMDA dose-dependently reducing expression in hippocampal slices, which is consistent with the effects of ketamine antagonism, but the contribution of GluN2B to this phenomenon is still being resolved as experimental conditions and choice of GluN2B antagonist produce different results (Ai et al., 2011; Spaethling et al., 2012; Vanhoose et al., 2006). In sum, these data demonstrate that ketamine enhances synaptic strength and excitatory output through AMPARs, which may occur through GluN2B-containing NMDARs, considering the similar effects of Ro25-6981, but these studies do not necessarily establish which cell type ketamine is acting on, nor how NMDAR blockade counterintuitively increases excitatory output. Importantly, there is no evidence that supports ketamine nor its metabolites binding or functionally acting on AMPARs directly (Shaffer et al., 2019).
The contribution of GluN2B containing NMDAR antagonism to the antidepressant-like effects as well as to the enhancement of excitatory signaling produced by ketamine is nuanced and still actively debated. Importantly, the GluN2B subunit confers the highest relative Ca2+ permeability among NMDAR subunits while also reducing deactivation rate and open probability (Paoletti et al., 2013). These biophysical characteristics allow GluN2B-containing NMDARs to potently enhance Ca2+ dependent intracellular mechanisms such as the activation of Ca2+ sensitive protein kinases. In contrast with the above findings, some studies observe that Ro25-6981 does not fully mimic the effects of ketamine or its metabolites. First, a study using rat hippocampal slices observed that ketamine (1 μM, 20-minute bath application) enhanced the action potential probability in CA1 measured through a disinhibition of pyramidal neurons (Widman & McMahon, 2018). Conversely, Ro25-6981 (1 μM, 20-minute bath application) does not significantly impact action potential probability and increases inhibitory currents. Intriguingly, the lack of an effect on action potential probability may be due to differential effects of Ro-256981 on individual cells, given that only a subset of cells showed an increase or showed a decrease following treatment. In contrast, a study using a genetic knockdown approach in mice found that loss of GluN2B in medial PFC (mPFC GABAergic interneurons, and specifically in those expressing somatostatin (SST) and parvalbumin (PV), occluded the antidepressant-like effects of ketamine in the forced swim test (Gerhard et al., 2020). The genetic manipulation alone appeared to reduce immobility relative to controls to a point where ketamine could not elicit a further reduction. This behavioral effect corresponded to a disinhibition of layer 5 mPFC pyramidal neurons whereby inhibition of GABA signaling strengthened pyramidal neuron activity. In another study using a genetic knockdown approach and in vivo Ca2+ imaging in mice, pyramidal neurons in the cingulate and secondary motor cortex were found to respond to ketamine (10 mg/kg) through an increase in Ca2+ event rate, particularly in dendritic spines, indicative of a hyperactive state which was facilitated by a reduction in Ca2+ events in SST interneurons (Ali et al., 2020). Additionally, this study observed that knockdown of GluN2B in SST interneurons mimicked the effects of ketamine on SST Ca2+ dynamics and pyramidal neuron dendrites. Overall, GluN2B in GABAergic interneurons may contribute to the antidepressant-like behavioral effects and strengthening of glutamatergic signaling effects of ketamine in the cortex, although it is not as clear in the hippocampus.
Several other studies have proposed that enhancement rather than antagonism of GluN2B-containing NMDARs is involved in producing antidepressant-like effects. First, a study in mice sought to understand how a NMDAR positive allosteric modulator AGN-241751 exerts antidepressant-like effects. AGN-241751 (50 μg/kg p.o.) was found to produce antidepressant-like effects in the forced swim and novelty suppressed feeding tests when administered 24 hours prior to testing (Pothula et al., 2021). These effects were dose-dependent, with higher doses (100 and 1000 μg/kg p.o.) failing to produce an effect. Similar antidepressant-like effects were observed when the mice were exposed to chronic unpredictable stress with additional positive effects in the sucrose splash and female urine sniffing tests. Additionally, this study found AGN-241751 to increase NMDAR-mediated inward currents in layer 5 mPFC pyramidal neurons in a dose-dependent, inverted “U” shaped manner (10 nM to 1 μM range) with a peak effect at 100 nM. Notably, AMPAR currents were not altered in the presence of 100 nM AGN-241751. Intriguingly, knockdown of GluN2B in pyramidal neurons, but not GABAergic interneurons, prevented the behavioral effects of AGN-241751 in the forced swim, novelty suppressed feeding, and sucrose splash tests. A similar study in mice which utilized rapastinel (GLYX-13), an NMDAR positive allosteric modulator, observed an increase in NMDAR-mediated currents in mouse layer 5 mPFC pyramidal neurons following rapastinel (0.1 but not 1.0 or 10 μM) treatment which required GluN2B expression in the pyramidal neurons (Pothula et al., 2020). Notably, glutamate release was not impacted by rapastinel. Behaviorally, this study also found that rapastinel (1 mg/kg) produced antidepressant-like effects in the forced swim (24 hours post-treatment), female urine sniffing (72 hours post-treatment), and novelty suppressed feeding tests (96 hours post-treatment). The behavioral effects of rapastinel were prevented by knockdown of GluN2B in pyramidal neurons, whereas this manipulation did not impact the ability of ketamine (10 mg/kg i.p.) to elicit antidepressant-like effects in the forced swim test. When GluN2B was knocked down in GABAergic interneurons, the antidepressant-like effects of rapastinel were maintained in the forced swim, female urine sniffing, and novelty suppressed feeding tests, whereas ketamine was unable to produce an effect in the forced swim test. These data suggest that positive allosteric modulation of NMDARs may be just as effective as antagonism in producing antidepressant-like effects in preclinical studies and their site of action (pyramidal neuron and GABAergic interneuron) differ. However, clinical trials for rapastinel (ClinicalTrials.gov Identifier: NCT03560518) revealed a lack of therapeutic efficacy relative to placebo whereas clinical trial results of AGN-241751 (NCT03726658, NCT03586427) remain to be posted. These studies also highlight how narrow the window of efficacy may be, as is also the case with ketamine, which will be important to consider as human studies continue.
The findings discussed above suggest that facilitation of AMPAR signaling by ketamine may be due to a disinhibition of synaptic glutamate signaling through inhibition of GABAergic interneurons. This disinhibition theory posits that ketamine blocks NMDARs on GABAergic interneurons, thus reducing their firing rate and removing the breaks on glutamatergic neurons (Grunze et al., 1996). In support, the NMDAR open channel blocker MK-801 decreases the firing of rapid spiking GABAergic interneurons which produces an increase in firing of pyramidal neurons in the rat PFC (Homayoun & Moghaddam, 2007). It was also reported that a single dose of ketamine (10mg/kg i.p.) in rats elicits its rapid (30 minutes) antidepressant-like effects in the forced swim test through impairing neuregulin 1 (NRG1) signaling in PV containing GABAergic interneurons which coincides with a reduction in PV and GAD67 (GABA synthesis enzyme) expression (Wang et al., 2014). Notably, PV is a Ca2+ binding protein that permits high firing rates, loss of which impairs the ability of interneurons to regulate neural networks via GABA release (Hu et al., 2014). Other studies have observed that GluN2A containing NMDARs in PV interneurons are necessary for the rapid actions of ketamine on evoked firing of visual cortex pyramidal neurons and gamma-band oscillations in mice (Picard et al., 2019). Predictably, another study observed a reduction in GABA and an increase in glutamate levels in the PFC and hippocampus following the forced swim test (about 36 minutes post-treatment), consistent with a disinhibition of synaptic glutamate signaling (Wang et al., 2014). A similar study in rats observed that a single dose of ketamine (10 mg/kg i.p.), which elicits rapid antidepressant-like effects in the forced swim test, coincided with a reduction in GAD67, PV, and GABA levels and an increase in glutamate levels 30 minutes post-administration in the PFC (Zhou et al., 2015). Collectively, these data lend support to the hypothesis that NMDAR blockade on GABAergic interneurons, especially those containing PV, may be involved in the mechanism of action of ketamine.
A study was conducted to determine if the rapid antidepressant-like effects of ketamine required NMDARs in PV interneurons (Pozzi et al., 2014). Transgenic mice (C57BL/6N background) with the GluN1 subunit genetically silenced were generated through crossing PV-Cre mice with GluN1 floxed (GluN1f/f) to generate PVCreGluN1 mice. In the forced swim test, ketamine (3 mg/kg) reduced immobility and increased swimming behavior in wild-type (WT) mice 30 minutes and 24 hours post-treatment. Conversely, ketamine did not alter immobility or swimming during the forced swim test 30 minutes, 24 hours, or one-week post-treatment in PVCreGluN1 mice relative to GluN1f/f mice which suggests that the antidepressant-like effects of ketamine require GluN1 in PV interneurons. However, saline treated PVCreGluN1 and GluN1f/f mice appear to have a 2- to 3-fold higher immobility and lower swimming time relative to C57BL/6N WT which suggests that the GluN1f/f mutation was alone sufficient to impact the FST. Given that the authors did not analyze the data across the WT and GluN1 genotypes, it is difficult to interpret the behavior of PVCreGluN1 mice. However, the authors assert that the effects of ketamine are unlikely to be fully explained by NMDARs in PV interneurons; a perspective that is gaining support. Effects of ketamine outside of NMDARs are further highlighted by advancements that demonstrate HNKs, which also have antidepressant-like effects in preclinical models, may not require NMDARs for their long-lasting effects on behavior or synaptic plasticity in the hippocampus (Gould et al., 2017; Riggs et al., 2020; Zanos et al., 2016).
The ketamine metabolite (2R,6R)-HNK has received significant attention due to its efficacy in rodent models of depression, while lacking psychotomimetic side effects and abuse potential (Zanos et al., 2016). Production of the 6-HNKs is important for the full antidepressant-like behavioral effects of ketamine (Zanos, Highland, Liu, et al., 2019; Zanos et al., 2016; Zheng et al., 2021). Importantly, the acute (one hour) and sustained (24 hour) antidepressant-like effects produced by (2R,6R)-HNK (10 mg/kg i.p.) in mice during the forced swim test are blocked by the AMPAR antagonist NBQX, akin to ketamine and Ro25-6981 as mentioned previously. However, the role of NMDARs in the mechanism of action of (2R-6R)-HNK is under active debate. The assertion that (2R,6R)-HNK does not require NMDARs was established by several studies that demonstrate its inability to displace MK-801 binding to NMDARs (10 μM). (2R,6R)-HNK has a binding affinity (Ki) and IC50 greater than 100 μM with 24% inhibition at 100 μM, and fails to impact NMDA-evoked currents in CA1 interneurons (Moaddel et al., 2013; Morris et al., 2017; Zanos et al., 2016). Likewise, structural analyses of the ketamine-NMDAR interaction are suggestive of HNKs having much lower potency in blocking NMDARs (Y. Zhang et al., 2021). However, the assertion that (2R,6R)-HNK does not impact NMDARs was challenged by a study which interrogated the effect of (2R,6R)-HNK on NMDAR-mediated miniature EPSCs (mEPSCs) in mouse hippocampal neuron cultures (Suzuki et al., 2017). This study found that NMDAR-mEPSCs were concentration-dependently and rapidly (within 10 minutes) attenuated by (2R,6R)-HNK with 10 μM having no effect and 50 μM producing an ~40% reduction in charge transfer. In contrast, AP5 (50 μM) and ketamine (50 μM) produced a 90-100% reduction in charge transfer indicating that AP5 and ketamine are more potent at inhibiting NMDAR-mEPSCs relative to (2R,6R)-HNK. Notably, Mg2+ was not present under these recording conditions which would otherwise compete for binding to the ion pore with ketamine or (2R,6R)-HNK. A subsequent study was conducted to investigate the concentration-dependent effect of (2R,6R)-HNK on NMDAR function (Lumsden et al., 2019). Using microdialysis, this study found that peak levels of (2R,6R)-HNK in the mouse hippocampus were 7.57 ± 2.13 μM 10 minutes following systemic administration of a dose (10 mg/kg i.p.) that results in antidepressant-like effects in several behavioral tests and returned to near baseline levels by two hours post-administration. Furthermore, in hippocampal slices, the newly established antidepressant-relevant concentration of (2R,6R)-HNK (10 μM) was unable to significantly impact NMDAR-mediated field excitatory postsynaptic potentials (fEPSPs), which were only inhibited by higher concentrations (200 μM) that are not behaviorally relevant. Additionally, the IC50 of (2R,6R)-HNK to inhibit NMDAR-mediated mEPSC amplitude (63.7 μM) was approximately 10-fold higher than that of ketamine (6.4 μM, Table 1). Intriguingly, (2S,6S)-HNK was markedly more potent at inhibiting NMDAR currents in the presence of glutamate and glycine in Xenopus oocytes in a subunit-specific manner relative to (2R,6R)-HNK. IC50 values for GluN2A, 2B, 2C, and 2D for (2S,6S)-HNK were 43, 21, 15, and 13 μM, respectively, whereas for (2R,6R)-HNK, the respective IC50 values were 498, 258, 202, and 287 μM. The inhibitory effects of (2S,6S)-HNK were enhanced at negative holding potentials, however, how effects of (2R,6R)-HNK may be impacted by holding potential was not explored due to the high IC50 values. Another study found that the inhibitory effects of (2R,6R)-HNK at a very high and pharmacologically not relevant concentration of 500 μM at GluN2A-containing NMDARs in HEK-293 cells in the presence of glutamate and glycine was enhanced by 84 and 87% at pH 6.8 and 7.2, respectively (Abbott & Popescu, 2020). In addition, this study found that association and dissociation rate constants for (2R,6R)-HNK were slower relative to ketamine. Furthermore, the inhibitory effects of (2R,6R)-HNK (500 μM) were enhanced at negative holding potentials, much like ketamine and Mg2+. Lastly, when (2R,6R)-HNK was applied prior to glutamate, the inhibitory effects on whole-cell NMDAR currents were increased relative to when it was co-applied with glutamate which suggests (2R,6R)-HNK preferentially binds inactive receptors. These findings demonstrate that pH, membrane potential, and the activity state of the NMDAR are all key variables that influence inhibition of NMDARs by (2R,6R; 2S,6S)-HNK, though their effective concentrations appear to be much higher than the antidepressant-relevant concentrations.
Ketamine and its metabolites have also been shown to interact with compounds that influence the function of group 2 metabotropic glutamate receptors (mGluR2/3) which have been implicated in depression. The mGluR2/3 receptor is inhibitory through its coupling to Gαi and consequent reduction in adenylate cyclase activity and cAMP production. It is most frequently localized to the presynaptic terminal and acts as an autoreceptor for glutamate that when activated inhibits the opening of voltage gated Ca2+ channels thus reducing vesicular release of neurotransmitter (Chavis et al., 1994; Shigemoto et al., 1997). Several preclinical studies have observed antidepressant-like effects of mGluR2/3 antagonists which align with the effects of ketamine (Chaki, 2017). First, a study tested an mGluR2/3 antagonist MGS0039 and observed a reduction in immobility and increased swimming during the forced swim test in mice within 24 hours of treatment with the mGluR2/3 antagonists MGS0039 (3 mg/kg i.p.) or LY341495 (1 mg/kg i.p.) (Chaki et al., 2004). In a similar behavioral test, the tail suspension test, MGS0039 and LY341495 both reduced immobility when administered one hour before the test. Furthermore, another study observed that LY341495 (3 mg/kg i.p.) in rats was sufficient to reverse anhedonia produced by a chronic unpredictable stress model of depression and measured using the sucrose preference test at 48 hours post-treatment (Dwyer et al., 2013). Considering these findings, mGluR2/3 antagonism appears to elicit antidepressant-like effects similar to ketamine, but the potential for a synergistic interaction with ketamine is unclear. A study in mice sought to address this question and found that both ketamine (10 mg/kg i.p.) and (2R,6R)-HNK (10 mg/kg i.p.) mimicked the reduction in immobility time in the forced swim test produced by LY341495 (3 mg/kg) 24 hours post-treatment (Zanos, Highland, Stewart, et al., 2019). Next, sub-effective doses of ketamine (1 mg/kg i.p.) and LY341495 (0.1 mg/kg i.p.), which were unable to alter immobility time alone, were able to significantly reduce immobility time when combined one-hour post-treatment, with a trend towards a reduction at 24 hours post-treatment. Likewise, a sub-effective dose of (2R,6R)-HNK (1 mg/kg i.p.) also synergized with LY341495 (0.1 mg/kg i.p.) to significantly reduce immobility time 24 hours post-treatment, with a trend towards a reduction at one-hour post-treatment. Expectedly, mGluR2/3 agonism via LY379268 (3 mg/kg) blocked the ability of ketamine and (2R,6R)-HNK (10 mg/kg i.p.) to reduce immobility. Finally, mGluR2 but not mGluR3 knockout mice had significantly less immobility time relative to WT mice while ketamine and (2R,6R)-HNK were incapable of further reducing immobility in the absence of mGluR2. Thus, these findings support antidepressant-like effects of mGluR2/3 antagonism which mimic the effects of and synergize with ketamine and (2R,6R)-HNK.
While the direct actions of ketamine metabolites on NMDAR function are still actively debated, there is also potential for indirect effects. In support, intracellular and extracellular D-serine levels in PC-12 cell cultures were reduced by (2R,6R; 2S,6S)-HNK with IC50 values of 0.68 ± 0.09 nM and 0.18 ± 0.04 nM respectively (Singh et al., 2016). The potential significance of this finding is that a lack of D-serine might preferentially impact GluN2B containing NMDARs as D-serine restricts their diffusion within membranes and reduces synaptic expression (Ferreira et al., 2017). However, these experiments will need to be repeated using neural cells to confirm the relevance to NMDAR function in the brain.
Collectively, these findings demonstrate that the mechanism of action of ketamine and its metabolite (2R,6R; 2S,6S)-HNK on the glutamate system, especially at the NMDAR, is complex. One of the key changes observed through NMDAR antagonism via ketamine, which largely targets GABAergic interneurons, is a strengthening of glutamatergic/AMPAR signaling which is necessary for antidepressant-like effects. It appears that (2R,6R)-HNK produces antidepressant-like effects and an enhancement in AMPAR signaling independent of NMDAR antagonism which suggests that NMDARs do not fully explain the mechanism of action of ketamine (Riggs et al., 2020; Zanos et al., 2016). These data also demonstrate that ketamine has a unique pharmacodynamic profile at the NMDAR, which is dependent on numerous variables such as subunit composition, presence of Mg2+, presence of co-agonists, membrane potential, pH, phosphorylation status, and cellular localization. Thus, it is important to consider how these variables may influence future studies and their translation into the clinic. Additionally, mGluR2/3 antagonists appear to synergize with and mimic the antidepressant-like effects of ketamine and (2R,6R)-HNK which is suggestive of a common route of action in the facilitation of excitatory signaling.
3. BDNF signaling
Brain derived neurotrophic factor (BDNF) is a peptide consisting of 118 highly conserved amino acids among vertebrate species that regulates cellular and molecular mechanisms of neuronal survival and synaptic plasticity in the adult brain and has therefore been implicated in several neuropsychiatric disorders (Autry & Monteggia, 2012; Duman et al., 2016; Götz et al., 1992; Radziejewski et al., 1992). BDNF can be stored in presynaptic terminals or dendrites and released in an activity-dependent manner. Once released, BDNF binds to its primary receptor Tropomyosin receptor kinase B (TrkB) (De Vincenti et al., 2019). TrkB is a receptor tyrosine kinase that dimerizes once BDNF is bound leading to autophosphorylation of its intracellular domains which act as docking sites for initiators of the mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and phospholipase C (PLC) pathways. These pathways are responsible for the litany of effects produced by BDNF including cellular growth and differentiation, transcription of synaptic proteins, cell survival, induction, and maintenance of LTP (Minichiello, 2009). BDNF signaling can be engaged by several neurotransmitter systems. Of particular interest to depression therapies, activation of serotonin or norepinephrine receptors has been shown to facilitate intracellular signaling pathways that increases expression of BDNF. Indeed, long-term treatment (21 days) with SSRIs or SNRIs, which enhance serotonin and norepinephrine signaling, increase the expression of BDNF mRNA in the hippocampus of rats (Nibuya et al., 1996). Consistent with this, norepinephrine dose-dependently increases BDNF expression in hippocampal neurons while also leading to the phosphorylation of TrkB (Chen et al., 2007). However, serotonin receptor agonists appear to have differential effects on BDNF expression depending on the brain region with significant decreases in the dentate gyrus of the hippocampus and increases in the parietal, frontal, and temporal cortex of rats (Vaidya et al., 1997). Likewise, chronic electroconvulsive seizure treatment can also increase BDNF mRNA expression in the hippocampus (Nibuya et al., 1995). Given these effects, the theory that disruption of neurotrophic factors such as BDNF could contribute to neuropsychiatric disease, especially depression, emerged (Duman et al., 1997).
The neurotrophic factor theory of depression, in part, emerged from several clinical and preclinical studies that showed significant gross structural changes in the hippocampus and PFC linked to neuronal atrophy (Deyama & Duman, 2020). Specifically, a series of magnetic resonance imaging (MRI) studies found that the left hippocampus of patients with remitted major depression had a 19% smaller volume (Bremner et al., 2000). Similarly, a respective 19% and 20% reduction in left subgenual PFC volume was found in young subjects (age 18-23) and older women (age 24-52) with major depression (Botteron et al., 2002). Furthermore, metabolism in the subgenual PFC, as measured by positron emission tomography (PET) scan, was reduced by 16.3% and 12.2% in bipolar and unipolar depression patients which corresponded to a 39% and 48% reduction in volume, respectively (Drevets et al., 1997). A more recent study built off these observations and found that the severity of depressive symptoms, as measured by the HAMD-17, inversely correlated with the synaptic density marker synaptic vesicle glycoprotein 2A (SV2A) labeled with the [11C]UCB-J radioligand and measured via PET scan (Holmes et al., 2019). This supports the notion that the loss of volume coincides with a loss in synapses in depressed patients. Importantly, reductions in brain volume were linked to disrupted BDNF signaling, as humans who carry a polymorphism in the BDNF gene (Val66Met) that disrupts BDNF secretion, had significantly lower hippocampal volumes which may put them at increased risk of developing depression (Egan et al., 2003; Frodl et al., 2007). Considering the importance of BDNF to the anatomical, cellular, and molecular changes occurring in depression, understanding how ketamine and its metabolites may influence changes in BDNF may help establish their complex mechanism of action (Duman et al., 2016).
The effects of ketamine on BDNF represents a key mechanism that appears to relate to the effects of ketamine and its metabolites which rapidly induce and maintain an antidepressant state. The cellular and molecular impact of ketamine as it relates to the strengthening of synaptic structures was established by (Li et al., 2010). This study demonstrated that systemic administration of ketamine (10 mg/kg i.p.) increased the expression of ARC, synapsin 1, PSD95, and GluA1, all key synaptic proteins, in the rat PFC 2 hours post-treatment. 24 hours later, EPSC amplitude at thalamocortical and intracortical synapses were enhanced. Another study provided further support to the idea that the synaptic structural changes induced by ketamine are critical for its sustained antidepressant effects (Moda-Sava et al., 2019). Using a chronic (21 day) corticosterone (CORT) exposure model of depression in mice, this study demonstrated that CORT induces dendritic spine elimination in the mPFC which is rescued 24 hours following a single ketamine treatment (10 mg/kg i.p.). Ketamine restored dendritic spines that were specifically lost during the CORT exposure. Behaviorally, ketamine rescued the increase in immobility produced by CORT exposure during the tail suspension test starting at three hours to one-week post-treatment. Importantly, the rapid antidepressant-like effects did not line up with the time course of spine formation, with observable increases by ketamine not occurring until 12 hours post-treatment. This suggests that the effect of ketamine on spine formation may not explain its rapid behavioral effects, rather they are responsible for maintaining these effects. Indeed, specific deletion of the newly formed spines 24 hours post-treatment via activated synapse-targeting photoactivatable Rac1 (AS-PaRac1), which collapses newly formed spines through light stimulation, reversed the effects of ketamine in the tail suspension test 48 hours post-treatment. Activation of AS-PaRac1 three hours post-treatment did not impact tail suspension test behavior 48 hours post-treatment, which is prior to the onset of spine formation by ketamine 12 hours post-treatment.
Considering that BDNF has been linked to the cellular and molecular mechanisms underlying synaptic strengthening and dendritic spine formation and ketamine in part mimics these effects, its mechanism of action may be to directly influence BDNF signaling (Duman et al., 2016). Indeed, a study in mice observed that ketamine (3 mg/kg i.p.) increased expression of BDNF protein in the hippocampus 30 minutes post-treatment (Autry et al., 2011). This effect corresponded with antidepressant-like effects in the forced swim test that were absent in BDNF knockout mice. Additionally, another study in rats found that infusion of an anti-BDNF antibody into the mPFC blocked the antidepressant-like effects of ketamine (10 mg/kg i.p.) in the forced swim test and ketamine treatment (0.5 μM) facilitated BDNF release from primary cortical neurons 15, 60, and 360 minutes post-treatment, and which was blocked by AMPAR antagonism (Lepack et al., 2014). In a follow up study, it was observed that incubation of primary rat cortical neurons with ketamine (0.5 μM) stimulated BDNF release which was blocked by the GABAA receptor agonist muscimol, which lends support to the hypothesis that glutamatergic, BDNF containing, neurons are disinhibited by ketamine acting on GABAergic neurons (Lepack et al., 2016). Furthermore, another study observed that (2S,6S)-HNK (10 mg/kg i.p.) increased BDNF release in the mPFC of mice 30 minutes post-treatment and (S)-ketamine (10 or 50mg/kg i.p.) dose-dependently increased BDNF release in the mPFC of mice 90 minutes post-treatment as measured by microdialysis (Anderzhanova et al., 2020). Additionally, another study sought to understand how ketamine and its metabolites influence BDNF signaling (Fukumoto et al., 2019). First, ketamine (10 mg/kg i.p.) and (2R,6R)-HNK (30 mg/kg i.p.) produced antidepressant-like effects in mice tested in the forced swim test and female urine sniffing test 24 hours post-treatment, and in the novelty suppressed feeding test three days post-treatment. Direct infusion of (2R,6R)-HNK (10 ng) into the mPFC produced similar effects in the forced swim and novelty suppressed feeding tests. The effects of (2R,6R)-HNK in the forced swim test and novelty suppressed feeding were blocked in mice containing the BDNF Val66Met allele (heterozygous Val/Met and homozygous Met/Met mice) (Fukumoto et al., 2019). Additionally, a BDNF neutralizing antibody infused into the mPFC blocked the effects of systemic (2R,6R)-HNK in the forced swim and novelty suppressed feeding tests which suggested that treatment was eliciting BDNF release in the mPFC. Indeed, (2R,6R)-HNK (10, 50 nM) stimulated BDNF release in cortical neuron cultures which was blocked by the AMPAR antagonist NBQX. These phenomena corresponded to an increase in the frequency and amplitude of hypocretin-induced EPSCs and amplitude of serotonin-induced EPSCs in layer 5 mPFC pyramidal neurons 24 hours post-systemic (2R,6R)-HNK (30 mg/kg i.p.) treatment (Fukumoto et al., 2019).
In further support of a link between ketamine and BDNF signaling, a study in mice observed that heterozygous and homozygous Val/Met and Met/Met mice had significantly reduced dendrite length, distal spine density, reduced spine diameter, and increased spine length (spine length not impacted in heterozygotes) in layer 5 mPFC pyramidal neurons relative to Val/Val WT controls (Liu et al., 2012). The effects of ketamine (10 mg/kg i.p.) on spine morphology 24 hours post-treatment, including increased spine number, increased diameter, and reduced length were significantly blunted but not completely abolished in Met/Met mice. These morphological changes corresponded to increased frequency of EPSCs induced by serotonin and hypocretin in slices from ketamine-treated mice which were blocked in Met/Met mice. Behaviorally, this study also observed that the antidepressant-like effects of ketamine in the forced swim test (reduced immobility) were absent in Met/Met mice. Given these findings, intact BDNF signaling is necessary for the cellular, molecular, and behavioral effects of ketamine in preclinical models. This study prompted an exploration of clinical data of ketamine’s efficacy, which revealed that Val/Val patients were more responsive 4 hours post-treatment (41% reduction in HAM-D score) to ketamine therapy relative to Met allele carriers (24% reduction in HAM-D score) (Laje et al., 2012). However, the findings from this study have been challenged due to the small sample size and a genome-wide association study that did not observe an association between Met allele carriers and ketamine efficacy 4 weeks post-treatment (Li et al., 2020). However, these authors concede that the differences in analysis timepoint in addition to the broader issue of the inability to replicate small studies of depression-linked genes warrant further investigation (Border et al., 2019). Furthermore, a study in humans found that (S)-ketamine (bolus of 0.11 mg/kg with 0.12 mg/kg maintenance i.v. infusion over 20 min) produced significant increases in overall hippocampal subfield volumes in healthy volunteers 65 minutes following treatment initiation independent of whether the volunteer carried the Met allele (Höflich et al., 2021). However, the authors of this study concede that the use of healthy volunteers likely influenced how the Met allele impacts the effects of (S)-ketamine. Certainly, more research is needed to determine the significance of the Val66Met allele among other potential genetic underpinnings of depression in humans and their response to ketamine therapy.
Ketamine and its metabolites also impact BDNF signaling in the midbrain which may be relevant for their antidepressant-like effects. A study using mouse midbrain dopamine neurons observed that ketamine (1 and 10 μM, 60-minute treatment) increased structural characteristics such as dendrite length, number, and soma area 72 hours post-treatment (Cavalleri et al., 2018). BDNF signaling through TrkB was necessary for these structural effects. These observations were also consistent with effects observed in human induced pluripotent stem cell (iPSC) derived dopamine neurons. Additionally, (2R,6R)-HNK (0.5 μM) resulted in increases in dendrite length, number, and soma area one- and six-hours post-treatment in mouse midbrain dopamine neurons and human iPSC dopamine neurons. In another study investigating effects of ketamine on the midbrain, (2R,6R)-HNK rapidly (within one hour, 10 mg/kg i.p.) reduced immobility in the forced swim test and increased sucrose preference in the sucrose preference test in rats using the chronic restraint stress model of depression (Chou et al., 2018). These behavioral effects persisted for 21 days post-treatment. 24 hours post-treatment, (2R,6R)-HNK restored AMPAR signaling in the ventrolateral periaqueductal gray (vlPAG) produced by the chronic restraint stress model of depression. The behavioral effects were replicated with direct microinjection of (2R,6R)-HNK (1 ng/0.2 μL) into the vlPAG which were blocked by the AMPAR antagonist CNQX. In a follow up study in rats, it was observed that (2R,6R)-HNK (10 mg/kg i.p.) reduced immobility in the forced swim test and increased sniffing in the female urine sniffing test while also increasing biting and aggressive behavior in the resident-intruder test (Chou, 2020). These behavioral effects corresponded to increases in BDNF expression in the vlPAG. These effects were stereoselective, as (2S,6S)-HNK did not mimic the antidepressant-like and aggressive behaviors nor did it increase BDNF as was observed with (2R,6R)-HNK. Furthermore, this study observed that direct infusion of either the TrkB antagonist K252a (10 pM/0.2 μl/day) or the mTOR inhibitor rapamycin (100 μM/0.2 μl/day) into the vlPAG for 7 days mimicked the depression-like behaviors in the sucrose preference, female urine, and forced swim tests as well as aggression in the resident intruder test conducted on days 5, 6, and 7 of treatment, respectively. BDNF knockdown via RNAi in the vlPAG had the same impact whereas direct BDNF infusion (0.2 ng/0.2 μl) into the vlPAG rapidly (within 30 minutes) produced antidepressant-like effects in the forced swim, female urine sniffing, sucrose preference, and tail suspension tests while inducing aggressive behavior in the resident intruder test. Finally, the rapid behavioral effects (30-60 minutes) of either vlPAG microinjection (1 pg/ 0.1 μL) or systemic administration (10 mg/kg) of (2R,6R)-HNK required intact BDNF signaling in the vlPAG. Given these findings, the antidepressant-like effects of (2R,6R)-HNK require BDNF signaling in the vlPAG, however aggression may be a negative side effect that could translate to clinical settings.
Several studies suggest that ketamine, its metabolites, and classical antidepressants may share a common mechanism of action through directly binding or influencing TrkB signaling. First, a study in mice observed that the tricyclic antidepressant imipramine (30 mg/kg i.p.) increased phosphorylation of TrkB (residue Y816) 30 minutes post-treatment in the PFC (Rantamäki et al., 2011). This effect was independent of BDNF, as the effect persisted in BDNF knockout mice. However, it did not occur in embryonic cortical or hippocampal neurons, suggesting that developmental timepoint and experimental conditions influence this phenomenon. Likewise, this study observed that fluoxetine (30 mg/kg i.p.) also increased phosphorylation of TrkB one-hour post-treatment in the hippocampus of WT and serotonin transporter knockout mice, indicating that the effects of fluoxetine on TrkB are independent of its influence on monoamine signaling. The authors argue that the lack of TrkB phosphorylation by antidepressants in vitro support a theory that they are not directly binding TrkB. Although, another possibility is that the immaturity of the cells used had confounded TrkB signaling in a way that was inconsistent with signaling in the adult brain. A follow up study by the same group observed that many antidepressants, including fluoxetine, imipramine, ketamine, and (2R,6R)-HNK disrupt the interaction between TrkB and the AP-2 protein complex that is involved in clathrin-dependent endocytosis of activated TrkB (Fred et al., 2019). This leads to an increase in surface expression of TrkB thus enhancing availability for rebinding BDNF and reengaging signaling cascades at the cell surface. The disruption of the TrkB-AP-2 complex by fluoxetine occurred even in a cell-free assay (independent of cellular systems), which suggested that fluoxetine may be directly binding to TrkB eliciting a conformational change that reduced AP-2 binding. Indeed, a concurrent study by the same group established how antidepressants may interact with TrkB to facilitate synaptic plasticity (Casarotto et al., 2021). First, the authors observed that cholesterol is an essential component in the function of TrkB, and is necessary for TrkB to engage downstream PLC activity. The authors proposed that cholesterol interacts with residue Y433F of TrkB to elicit its effects. Importantly, fluoxetine, ketamine, and (2R,6R)-HNK facilitated PLC activity in cortical neurons through TrkB which was prevented by cholesterol sequestration. Given this, it was hypothesized that fluoxetine, ketamine and (2R,6R)-HNK may act by directly binding to the transmembrane domain of the TrkB receptor. Indeed, immunoprecipitation of TrkB with biotinylated fluoxetine revealed a direct interaction. Ketamine and (2R,6R)-HNK could displace binding of fluoxetine to TrkB which suggests that they are also capable of binding TrkB. (2S,6S)-HNK was much less capable at displacing fluoxetine which suggests that binding to TrkB is stereoselective for (2R,6R)-HNK. Furthermore, the addition of cholesterol enhanced binding of fluoxetine and (2R,6R)-HNK to TrkB, which may have important implications for their pharmacodynamics. The behavioral significance of this finding was that both ketamine and (2R,6R)-HNK (10 mg/kg) induced a hyperplastic state in the visual cortex of adult mice that allowed for a shift in ocular dominance which otherwise can only occur early in development. Additionally, mutation of the putative transmembrane binding site of TrkB (residue Y433) prevented ketamine’s actions (10 mg/kg) in the forced swim test (treatment two hours before), and extinction of conditioned fear (treatment immediately post-conditioning and two hours prior to extinction sessions). Collectively, these studies highlight the nuance associated with observing the interaction between antidepressants and TrkB; while the initial reports made by Rantamäki et al. (2011) hypothesized that a direct interaction was unlikely to occur, it is possible that the in vitro conditions of those experiments were less permissive to observing an interaction due to a lack of sufficient cholesterol to facilitate binding. In support of this interpretation, the primary embryonic cultures used in that study lacked astrocytes which serve as critical sources of cholesterol for neurons to form mature synapses (Mauch et al., 2001). Intriguingly, one study observed an increase in cholesterol mobilization in the plasmalemma of astrocytes following acute ketamine (2.5 and 25 μM for 30 minutes) exposure which may enhance astrocyte to neuron cholesterol transfer, thus facilitating TrkB signaling and synaptic maturation (Lasič et al., 2019). There remains much work to be done to explore these phenomena and if these results are replicated, they would suggest that direct interaction with the TrkB receptor may underlie a common mechanism of action for antidepressants.
Collectively, these studies demonstrate that BDNF signaling is critical for the cellular, molecular, and behavioral effects of ketamine and its metabolites. As discussed in the previous section, ketamine and its metabolites can enhance AMPAR signaling through a disinhibition mechanism involving either GABAergic interneuron NMDARs or inhibitory extrasynaptic NMDARs in pyramidal neurons. Considering that AMPARs drive BDNF secretion, these proposed disinhibition mechanisms produced by ketamine and its metabolites are likely needed to trigger BDNF effects (Jourdi et al., 2009). However, given the HNKs have much less impact at NMDARs, it begs the question as to how they trigger BDNF secretion or signaling. This highlights the importance of the findings by Casarotto et al. (2021) which, if replicated, suggest that (2R,6R)-HNK directly binds to and triggers TrkB signaling to enhance synaptic plasticity. Thus, the lack of abuse potential and psychotomimetic effects observed with (2R,6R)-HNK relative to ketamine in rodents may be due to this mechanism, as NMDAR disruption is greatly reduced but the structural bolstering of synapses is maintained via TrkB.
4. Opioid system
The endogenous opioid system is widely studied for pain management. Opioids were also used to treat depression up until the 1950s, but this therapeutic strategy quickly shifted in favor of the less addictive monoamine oxidase inhibitors and tricyclic antidepressants (Tenore, 2008). However, research into disruptions of the opioid system in depression, especially as it relates to psychological or emotional pain, and the development of less addictive opioid system-targeting compounds has been reinvigorated (Peciña et al., 2019). The opioid system consists of four G protein-coupled receptors: μ, κ, δ, and nociceptin and three peptide families: β-endorphins, enkephalins, and dynorphins. While these peptides and receptors are well known for their analgesic properties, they are also involved in the stress response, regulation of mood, and reward/motivation which all contribute to the depression phenotype. Indeed, both preclinical and clinical studies support a role for the opioid system in both producing and alleviating depressive-like states. Specifically, agonism of κ-opioid receptors were shown to produce depression-like symptoms in humans (Pfeiffer et al., 1986). Likewise, this study also observed that κ-opioid receptor agonism increased immobility, i.e., induced a depression-like phenotype, in the forced swim test in rats whereas antagonism decreased immobility. Furthermore, buprenorphine, which is a partial μ-opioid receptor agonist and potent κ-opioid receptor antagonist rescues the increase in immobility in the forced swim test produced by an unpredictable chronic mild stress model of depression in mice (Falcon et al., 2016). Ketamine has also been used for pain management, especially for neuropathic pain which can involve upregulation of NMDARs in ascending central pain circuitry and/or loss of top-down (executive) regulation of pain (Niesters et al., 2014). Under these circumstances, ketamine counters the hyper-excitatory state present in ascending pain circuitry through NMDAR inhibition while also enhancing the activity of brain regions involved in top-down inhibition of pain centers such as the PFC and brainstem (Niesters et al., 2012). It is possible that these same mechanisms may be involved in dampening psychological pain. Furthermore, the analgesic effects of ketamine in mice are attenuated by antagonism of μ and δ opioid receptors via naloxone, clocinnamox and naltrindole which suggests that ketamine and opioids likely overlap mechanistically in modulating pain perception (Pacheco Dda et al., 2014).
Given the involvement of the opioid system in depression, it was hypothesized that the human antidepressant effects of ketamine may involve the opioid system. An initial small clinical study (n = 12) tested the hypothesis that the opioid system was necessary for the antidepressant effects of ketamine in humans with treatment resistant depression (Williams et al., 2018). This study found that the opioid receptor antagonist naltrexone (50 mg) attenuated the antidepressant effects of ketamine (0.5 mg/kg i.v. over 40 minutes) one to three days post-treatment as measured by the Hamilton Depression Rating Scale 6 item and 17 item. Notably, naltrexone did not impact the dissociative symptoms of ketamine. Data collected by this study requires careful interpretation as argued by several response articles to this study. These criticisms range from the low sample size, a lack of a naltrexone only control, the saturating dose of naltrexone given, and the target population used for the study excluding substance abusers (Amiaz, 2019; George, 2018; Heifets, Williams, et al., 2019b). Conversely, others argued that the data are more suggestive of ketamine, and even placebo effects, requiring an intact endogenous opioid system, not necessarily exogenous stimulation of opioid receptors via ketamine, which was broadly agreed upon by the original authors (Heifets, Williams, et al., 2019a; Sanacora, 2019). In support of this interpretation, a study using congenitally learned helpless (cLH) rats observed increased Ca2+ signaling in the lateral habenula (LHb), a brain region involved in negative affective regulation and implicated in depression, relative to WT rats (Klein et al., 2020). This effect was reduced by ketamine application to LHb slices (10 μM for 15 minutes). The increased activity in the LHb correlated with increased immobility in the forced swim test. Forced swim test performance was then assessed in cLH rats that received ketamine (15 mg/kg i.p. two hours pre-test), naltrexone (1 mg/kg s.c. one hour prior to ketamine) or a combination. Whereas naltrexone blocked the antidepressant-like effects of ketamine in the forced swim test, it had no impact on immobility on its own. Additionally, this study observed that ketamine improved motivation for obtaining a sucrose reward in the progressive ratio test one day post-treatment which was prevented by naltrexone. These behavioral effects correlated with naltrexone preventing the ketamine-induced reduction in Ca2+ intensity seen in LHb slices, with naltrexone having no effect on its own. To test for the sufficiency of the opioid system to produce antidepressant-like effects, morphine (10 mg/kg s.c.) was administered prior to the forced swim test but did not produce the same reduction in immobility as ketamine. Expectedly, morphine and the μ opioid receptor agonist DAMGO did not influence Ca2+ intensity in LHb slices. Lastly, this study found that cLH rats did not develop a condition place preference to ketamine but did to morphine as measured using the conditioned place-preference test. Overall, these findings suggest that the opioid system is necessary, but not sufficient on its own, for the antidepressant-like effects of ketamine.
Another study in mice found that naltrexone (2 mg/kg i.p. 30 minutes prior to ketamine) prevented the reduction in immobility produced by ketamine (10 mg/kg i.p.) in the tail suspension test both 30 minutes and 24 hours post-treatment (F. Zhang et al., 2021). Additionally, naltrexone prevented the antidepressant-like effects of ketamine at 30 minutes but not at 24 hours post-treatment in the differential-reinforcement-of-low-rate 72 s paradigm. Notably, this operant task is designed to assess temporal discrimination and impulsivity and has predictive validity for screening antidepressant drugs. However, ketamine only transiently impacts behavior (interpreted as reduced impulsivity/improved temporal discrimination) in this task when given acutely (Hillhouse & Porter, 2014). This may not be indicative of the antidepressant-like effects of ketamine due to the transient nature of the effect and may instead be due to altered temporal discrimination which has been documented in humans treated with ketamine and which are associated with psychotomimetic effects (Coull et al., 2011; Moore et al., 2013). Collectively, these studies lend support to the necessity of the opioid system for the antidepressant effects of ketamine in humans and rats, but direct engagement of the opioid system does not appear to sufficiently explain the mechanism of action of ketamine.
The assertion that the opioid system is required for the antidepressant effects of ketamine has been challenged experimentally. In support, a study in mice exposed to 10 days of social defeat stress observed that naltrexone (10 mg/kg i.p. 30 minutes prior to ketamine) did not block the antidepressant-like effects of ketamine (10 mg/kg i.p.) in the tail suspension test (3 hours post-ketamine), forced swim test (1 day post-ketamine), or sucrose preference test (three days post-ketamine) (Zhang & Hashimoto, 2019). Similarly, mice exposed to lipopolysaccharide injection stress one day prior to testing manifested greater immobility in the forced swim test (three hours post-ketamine) that was rescued by ketamine, an effect which was not blocked by naltrexone. Additionally, a small study conducted in patients with comorbid major depression and alcohol use disorder (n = 5) found that pretreatment with a single dose of naltrexone (380 mg intramuscular [i.m.] two to six days prior to the first ketamine infusion) did not impact overall improvement of depression symptoms (MADRS score) over the course of four ketamine infusions (0.5 mg/kg) spread across four weeks (Yoon et al., 2019). The findings of this study were challenged (Heifets, Williams, Bentzley, et al., 2019). A concern was the open-label design, which likely contributed to expectancy bias and placebo effects. Additionally, the peak naltrexone levels with i.m. delivery would occur two days post-administration, yet ketamine was delivered two to six days later. Lastly, considering the sample population of patients, which had alcohol use disorder, it has been found naltrexone produces an antidepressant effect in this population which likely explains why naltrexone didn’t impair the effects of ketamine (Adamson et al., 2015; Pettinati et al., 2010). In response, others articulate that the argument that naltrexone levels were likely too low to interact with ketamine are paradoxical when considered with the assertion that naltrexone levels were sufficient to produce an antidepressant effect on its own (Krystal et al., 2019). Furthermore, the studies cited do not provide evidence for an antidepressant effect of naltrexone alone in their primary analyses. Another small study conducted in patients with moderate to severe treatment-resistant depression had similar findings (Marton et al., 2019). Specifically, a group of patients that were regularly taking buprenorphine and methadone (μ opioid receptor agonists) for over a year were administered a ketamine regimen (0.5 mg/kg over 40 minutes twice per week for three weeks) and their Beck Depression Inventory-II (BDI II) scores were recorded. Relative to controls, buprenorphine- and methadone-taking patients did not differ in the improvement of their BDI II scores following ketamine treatment. The single patient that was taking naltrexone also had similar improvement. Given these findings, the assertion that ketamine overlaps mechanistically with the opioid system to elicit its antidepressant effects remains controversial and requires further investigation. As stated previously, these small studies can be difficult to replicate and may not be representative of a broader population.
The strength of interaction between ketamine and its metabolites with opioid receptors are stereoselective and vary depending on the receptor (Zanos et al., 2018). The possibility that ketamine may be directly interacting with opioid receptors began as a controversial topic, with some studies observing or failing to observe displacement of opioid receptor ligands by ketamine (Finck & Ngai, 1982; Fratta et al., 1980; Smith et al., 1980). A growing body of evidence has since supported an interaction between ketamine and opioid receptors. First, a study using guinea pig brain homogenate observed that the binding affinity of ketamine at opioid receptors is stereoselective (Table 2), with (S)-ketamine having a higher affinity for μ, κ, and δ opioid receptors (Ki = 11, 24, and 130 μM, respectively) over (R)-ketamine (Ki = 28, 100, and 130 μM, respectively) (Hustveit et al., 1995). Another study reported similar observations that ketamine interactions are stereoselective for recombinant human opioid receptors expressed in Chinese hamster ovary cells (Hirota et al., 1999). Specifically, (S)-ketamine had a greater affinity for μ, κ, and δ opioid receptors (Ki = 28.6, 23.7, and 205 μM respectively) relative to (R)-ketamine (Ki = 83.8, 60, and 286 μM respectively, Table 2). Likewise, similar range of affinity values at μ and κ opioid receptors were recently reported for (S)-ketamine (μ: Ki = 7 μM and κ: Ki = 19 μM) and (R)-ketamine (μ: Ki = 14 μM and κ: Ki = 40 μM) (Bonaventura et al., 2021). Additionally, this study showed that (S)-ketamine acted as a partial G protein signaling agonist at μ opioid receptors (EC50 = 9 μM, Emax = 48%) and as a full agonist at κ opioid receptors (EC50 = 16 μM, Emax = 84 %) whereas (R)-ketamine acted as a weak partial agonist at both μ opioid receptors (EC50 = 34 μM, Emax = 39 %) and κ opioid receptors (EC50 > 100 μM, Emax < 50%). Interestingly, neither enantiomer activated ß-arrestin. Furthermore, computational analyses of the predicted interaction between ketamine and its metabolites to the μ and κ-opioid receptor suggested that (2R,6R)-HNK interacted with the orthosteric binding pockets at high affinity (Joseph et al., 2021). This study also used G-protein recruitment assays to conclude that (2R,6R)-HNK acts as an inverse agonist, with IC50 values of 0.56 nM and 2.1 x 10−5 nM for the μ and κ-opioid receptor, respectively. Maximum inhibition of G-protein recruitment at the μ and κ-opioid receptor was achieved at 10 nM (2R,6R)-HNK. Intriguingly, ketamine was largely ineffective at eliciting an inverse agonist effect at μ and κ-opioid receptors even though their computational prediction suggested that it interacted with these receptors with high affinity. The effect of (2R,6R)-HNK was blocked by pretreatment with the opioid receptor antagonist naltrexone, which is consistent with previously discussed findings in humans and rodents that the antidepressant effects of ketamine are blocked by naltrexone. Given these data, it is possible that the antidepressant effects of ketamine that overlap with the opioid system involve its principal metabolite (2R,6R)-HNK. However, this study’s main findings rely on computational analysis and the predicted affinity and G protein recruitment values it reports are not consistent with several prior publications (Bonaventura et al., 2021; Hirota et al., 1999; Nemeth et al., 2010).
Table 2: Direct activity of ketamine at opioid receptors.
The binding affinity (Ki) of ketamine to opioid receptors is stereoselective with (S)-ketamine having higher affinity relative to (R)-ketamine with both enantiomers favoring binding to μ and κ over δ-opioid receptors. Functionally, (S)-ketamine is a partial and full agonist at μ and κ-opioid receptors, respectively. (R)-ketamine is a weak partial agonist at μ and κ-opioid receptors. Experiments were conducted using hamster ovary cells (Hirota et al., 1999), guinea-pig brain (Hustveit et al., 1995), or rat brain tissue (Bonaventura et al., 2021; Nemeth et al., 2010). No studies to date have reported Ki of binding of (2R,6R)-HNK to opioid receptors. EC50, half maximal effect concentration; Emax, maximal effect at receptor saturation.
| Drug | Opioid Receptor |
Effect | References |
|---|---|---|---|
| (S)-ketamine | μ | Ki=7-28.6 μM; EC50=7-9 μM; Emax=48% | (Hustveit et al., 1995; Hirota et al., 1999; Bonaventura et al., 2021) |
| κ | Ki=19-24 μM; EC50=16 μM; Emax=84% | ||
| δ | Ki=130-205 μM | ||
| (R)-ketamine | μ | Ki=14-83.8 μM; EC50=34 μM; Emax=39% | (Hustveit et al., 1995; Hirota et al., 1999; Bonaventura et al., 2021) |
| κ | Ki=40-100 μM; EC50>100 μM; Emax<50% | ||
| δ | Ki=130-286 μM | ||
| (R,S)-ketamine | μ | Ki=4.38-42.1 μM | (Hustveit et al., 1995; Hirota et al., 1999; Nemeth et al., 2010) |
| κ | Ki=25-28.1 μM; EC50=29 μM | ||
| δ | Ki=272 μM |
The mechanistic overlap between pain, reward processing and antidepressant effects is potentially problematic for therapeutic intervention with ketamine. Estimates suggest that 51.4% of the opioid prescriptions in the United States are received by the 16% of Americans who have a mood or anxiety disorder, which is suggestive of patients seeking to rectify a negative affective or emotionally painful state through opiate use (Davis et al., 2017). The comorbidity of substance use and depression raises concerns that antidepressant therapies such as ketamine are likely to engage neural systems involved in reward processing and addiction, such as the opioid system. Indeed, one of the major criticisms of therapeutic use of ketamine is that it has significant abuse potential due to its rewarding properties. However, some observations raise the possibility that ketamine does not carry the same degree of hedonic value as morphine in a learned helplessness model of depression (Klein et al., 2020). Additionally, (S)-ketamine induces conditioned place preference in mice and conditioned operant lever responding for an infusion in rats whereas (R)-ketamine largely does not which suggests (S)-ketamine may have greater abuse potential (Bonaventura et al., 2021). While some studies support this conclusion, at sufficient doses (R)-ketamine does induce a conditioned place preference, as well as locomotor stimulation, in mice (Yang et al., 2020; Zanos, Highland, Liu, et al., 2019). The Bonaventura et al. (2021) study also observed conditioned lever responding for (S)-ketamine is rapidly extinguished with fewer than 10 responses over three hours on the first extinction training day, indicative of a lack of compulsive drug seeking. Given this, the abuse potential of (S) and in particular (R)-ketamine appears to be much lower than anticipated, especially compared to stimulants and opioids like cocaine and morphine (Venniro et al., 2020). Thus, the clinical or recreational/self-medicating use of ketamine is perhaps unlikely to result in substance abuse disorder. Additionally, there is evidence that (2R,6R)-HNK does not elicit the same desire to self-administer or hyperlocomotion effects as ketamine although it is not currently clear whether (2R,6R)-HNK impacts the opioid system in a way that reduces its abuse potential (Highland et al., 2019; Zanos et al., 2016).
Collectively, these studies illustrate that the interaction between ketamine and the opioid system as it relates to antidepressant effects is complex and controversial. Current clinical studies are difficult to interpret due to low sample sizes and experimental controls. The current predominant theory according to these studies is that ketamine, and possibly the placebo effect, require a functional opioid system to achieve their antidepressant effects (Krimmel et al., 2020). The preclinical data supports this, in part, but these studies also have methodological inconsistencies such as the timing and dose of naltrexone administration relative to ketamine. Intriguingly, (R) and (S)-ketamine appear to have agonist properties at μ and κ opioid receptors, with (S)-ketamine having a higher affinity whereas (2R,6R)-HNK may be an inverse agonist at μ and κ-opioid receptors within antidepressant relevant concentrations, though these findings require replication. The therapeutic relevance of these findings remains to be fully elucidated in human populations, but they may provide a mechanistic explanation for the differences in willingness to self-administer (R,S)-ketamine over (R)-ketamine and (2R,6R)-HNK in preclinical studies.
5. Monoamine signaling
The monoamine hypothesis has been the predominant theory for the etiology of depression following the discovery of monoamine-acting antidepressants in the 1950s. The first drugs used in clinical trials to treat depression resulted from the serendipitous discovery in the early 1950s that iproniazid, a drug used to treat tuberculosis, induced an “euphoric” state in patients, which was initially viewed as an adverse effect of the drug, but nonetheless led to its use in debilitated patient groups. It was subsequently observed that iproniazid inhibited human monoamine oxidase, and the drug was therefore proposed to improve depressive symptoms through preventing the metabolism of serotonin, dopamine, and norepinephrine (Sandler, 1990). Following these observations, there was identification of compounds that target the monoamine systems such as SSRIs, SNRIs, and tricyclic antidepressants which collectively impair the clearance of monoamines from the extracellular space by blocking the serotonin transporter (SERT) and norepinephrine transporter (NET) (Ferguson, 2001). The endogenous source for the majority of monoamines in the forebrain come from the midbrain, with serotonin, norepinephrine, and dopamine arising from the dorsal raphe (DR), locus coeruleus (LC), and ventral tegmental area (VTA) or substantia nigra pars compacta, respectively (Bromberg-Martin et al., 2010; Itoi & Sugimoto, 2010; Michelsen et al., 2008). There are a number of receptors that are sensitive to the monoamines, with five dopamine receptors (D1-5), three norepinephrine receptors each with three subtypes (α1a,b,d, α2a,b,c and β1,2,3) and seven serotonin receptor families (5-HT1-7) comprised of 14 total receptors all of which are G-protein coupled, except for 5-HT3 (Beaulieu & Gainetdinov, 2011; Nichols & Nichols, 2008; Strosberg, 1993). Critically, while monoamine-targeting compounds have been shown to be effective in some populations, many patients require multiple treatment steps to achieve remission of their depression symptoms (Rush et al., 2006). In the population of patients that are resistant to monoamine system targeting therapies, ketamine has been shown to facilitate remission, hence the current indication by the FDA (Lapidus et al., 2014). Given this, there is interest in how ketamine and its metabolites may interact with serotonin, dopamine, and norepinephrine as it may reveal how it can overcome treatment resistance.
The impact of ketamine and its metabolites on the concentration of extracellular monoamines in the PFC has been characterized. A microdialysis study in mice investigated the impact of ketamine enantiomers and their metabolites on extracellular serotonin, dopamine, and norepinephrine in the PFC of mice (Ago et al., 2019). For serotonin, (R)-ketamine (10 and 20 mg/kg i.p.) produced an increase at 20-, 40-, and 60-minutes post-treatment with a peak effect size at ~275% of baseline following a 20 mg/kg dose at 20 minutes. (S)-ketamine (20 mg/kg) produced an increase at 20,40, and 60 minutes with a peak effect size at ~175% of baseline with the 10 mg/kg dose lacking an effect at 60 minutes. (2R,6R)-HNK (20 mg/kg) increased serotonin at 40- and 60-minutes post-treatment with a peak effect at ~140% of baseline whereas (2S,6S)-HNK and (R) and (S)-norketamine (20 mg/kg) were ineffective. For dopamine, (R)-ketamine (20 mg/kg) resulted in only modest changes, with a modest increase 40 minutes post-injection. Conversely, (S)-ketamine (20 mg/kg) produced a large increase (~240% of baseline) 40 minutes post-treatment with a significant increase maintained following 120 minutes. (R) and (S)-norketamine (20 mg/kg) shared a ~150% peak increase at 60 minutes that was largely maintained following 120 minutes. (2R,6R;2S,6S)-HNK (20 mg/kg) did not result in a change in dopamine. Additionally, norepinephrine levels were significantly impacted by (R)-ketamine (20 mg/kg) with a peak effect size of ~160% of baseline 40 minutes post-treatment and remained heightened following 120 minutes, like the 10 mg/kg dose. (S)-norketamine (20 mg/kg) produced a peak effect size (~150% of baseline) 40 minutes post-treatment whereas (R)-norketamine was ineffective. (2R,6R)-HNK but not (2S,6S)-HNK produced a transient peak effect (~160% of baseline) at 20 minutes which disappeared following 60 minutes. The peak increases in dopamine by (R)-ketamine and peak increases in serotonin and dopamine by (S)-ketamine are prevented by the AMPAR antagonist NBQX pretreatment 20 minutes prior. These data demonstrate that the dynamic fluctuations in extracellular monoamines in the PFC of mice produced by ketamine and its metabolites are stereoselective and concentration dependent which highlights the complexity of the pharmacodynamics of ketamine.
Another microdialysis study observed increases in extracellular serotonin and norepinephrine in the mPFC but not in the DRN of rats two hours following treatment with ketamine (25 mg/kg, i.p.) (López-Gil et al., 2019). Extracellular glutamate was also increased in the mPFC and the DRN two hours post-ketamine. Reverse dialysis of ketamine (300 μM, 1 μL/min) into the mPFC over a span of two hours replicated the increases in norepinephrine, serotonin, and glutamate in the mPFC but also produced an increase in glutamate within the DRN. These data suggest that ketamine facilitates activation of glutamatergic projection neurons in the mPFC to the DRN and LC which drives serotonin and norepinephrine release in the mPFC. Another study in mice found that direct infusion of ketamine (0.25 μg/side) into the mPFC was sufficient to produce antidepressant-like effects in the forced swim test (Pham et al., 2017). The observed increase in swimming behavior was significantly correlated with an increase in extracellular serotonin in the mPFC. AMPARs in the DRN were necessary for these effects as antagonism prevented the antidepressant-like effects and increase in mPFC serotonin. Another study in mice observed antidepressant-like effects of ketamine in the forced swim test which mimicked the effects of the mGluR2/3 antagonist LY341495 (Fukumoto et al., 2016). This occurred when ketamine or LY341495 were systemically administered (30 mg/kg and 3.0 mg/kg i.p. respectively) or directly infused into the mPFC (3 nmol/0.1 μL/side and 0.03 pmol/0.1 μL/side respectively). Systemic (10 mg/kg s.c.) or direct infusion (0.03 nmol/side) of the AMPAR antagonist NBQX into the mPFC blocked the antidepressant-like effects in the forced swim test. These behavioral effects corresponded to an increase in activity of serotonin neurons in the DRN as measured by c-Fos immunoreactivity which was blocked by NBQX. Lastly, a study in rats observed that the acute and sustained antidepressant-like effects of (S)-ketamine (15 mg/kg) in the forced swim test one and 48-hours post-treatment are prevented by serotonin depletion via pretreatment with an irreversible tryptophan hydroxylase inhibitor para-chlorophenylalanine (pCPA) (du Jardin et al., 2017). The acute and sustained effects of (S)-ketamine were rescued by a 5-HT1B receptor agonist CP94253 administered two hours prior to the forced swim test. Collectively, these microdialysis and behavioral pharmacology experiments lend support to the antidepressant-like mechanism of action of ketamine involving serotonin and norepinephrine release into the PFC.
Several clinical and pre-clinical studies have investigated the impact of ketamine on the binding of serotonin receptors and transporters. For example, SERT occupancy was assessed in a PET study following ketamine infusion (0.5 mg/kg i.v. over 40 minutes) in healthy volunteers using the radioligand [11C]DASB (administered five minutes post-ketamine) (Spies et al., 2018). This study did not observe significant occupancy of ketamine at SERT in the caudate, putamen, thalamus, and whole brain ROIs. In another PET study, the impact of ketamine (0.5 mg/kg i.v. over 40 min) on 5-HT1B receptor binding in SSRI resistant patients with major depression was assessed using the radioligand [11C]AZ10419369 (Tiger et al., 2020). A significant increase in 5-HT1B receptor binding (16.7%) was observed in the hippocampus following a single ketamine treatment. In a similar study using rhesus monkeys, SERT occupancy and 5-HT1B receptor binding was assessed via PET following ketamine treatment (30 mg/kg i.v. bolus with a 7.5mg/kg/hr) maintenance infusion through the scan) 100 minutes prior (Yamanaka et al., 2014). Using the radioligand [11C]AZ10419369, 5-HT1B receptor binding was found to be significantly increased in the nucleus accumbens, ventral globus pallidus, and the midline nucleus reuniens of the thalamus which was blocked by AMPAR antagonism. Reduced SERT occupancy was also observed using the radioligand [11C]DASB in these brain regions in addition to the occipital cortex and lateral geniculate nucleus. In another study, the antidepressant-like effects of ketamine (32 mg/kg i.p.) were absent in mice lacking SERT or plasma membrane monoamine transporter (PMAT) (Bowman et al., 2020). These behavioral effects corresponded to a reduction in serotonin clearance in the CA3 region of the hippocampus in WT mice but not SERT or PMAT knockout mice. This suggests that the antidepressant-like effects of ketamine are impacted by loss of SERT and PMAT, but not necessarily that ketamine requires either transporter to elicit its effects making these transporters unlikely direct targets of ketamine. Collectively, these data suggest that ketamine positively influences serotonin signaling through enhancing binding of 5-HT1B receptors but not necessarily through directly binding and inhibiting SERT.
A series of electrophysiological and microdialysis experiments have established a link between an antidepressant dose of ketamine and enhancement of dopamine signaling in key brain regions involved in depression (Kokkinou et al., 2018). First, a study in rats observed that ketamine (10 mg/kg i.v.) increased the number of spontaneously firing dopamine neurons in the VTA when administered 10 minutes prior to recordings (Witkin et al., 2016). This effect corresponded to a peak increase of over 300% in extracellular dopamine in the mPFC within 10 minutes of ketamine treatment (10 mg/kg s.c.) relative to baseline. A similar effect of ketamine (25 mg/kg i.p.) was observed in the NAc with a peak increase of over 150% of baseline. In another study, which utilized the learned helplessness-susceptible Wistar-Kyoto rat line, it was found that ketamine (5 mg/kg i.p.) rescued the learned helplessness phenotype, i.e. had fewer escape failures and reduced latency to escape, when administered 20 minutes or 24 hours prior to an active avoidance task (Belujon & Grace, 2014). These behavioral effects corresponded to a reduction in dopamine neuron activity in the VTA in helpless rats which was rescued by ketamine 20 minutes post-treatment. When administered 24 hours prior to recordings, ketamine rescued a decrease in the number of spontaneously firing dopamine neurons and rescued a reduction in their firing rate in learned helpless rats. Furthermore, this study also observed that ketamine restored LTP induction which was otherwise impaired in helpless rats in the hippocampus to nucleus accumbens core (NAcc) pathway when administered 20 minutes prior to induction. Notably, this effect was blocked by infusion of a D1 receptor antagonist, which suggests that ketamine may be enhancing dopamine release, thus activating D1 receptors, to facilitate LTP.
In contrast to the above studies, the impact of ketamine on dopamine release parameters in the NAcc were investigated using fast scan cyclic voltammetry in mice in anesthetized mice (Can et al., 2016). Ketamine (2, 10, or 50 mg/kg i.p.) failed to alter the rise time, decay constant, or peak amplitude of evoked dopamine release from VTA afferents into the NAcc. Furthermore, an in vitro receptor affinity screen using HEK293 cells revealed that (S) and (R)-ketamine and their metabolites (S)-norketamine, (R)-norketamine, (S)-DHNK, (R)-DHNK, (2S,6S)-HNK, (2R,6R)-HNK, (2R,6S)-HNK, and (2S,6R)-HNK (10 μM) had little to no ability to bind or functionally agonize or antagonize D1-5 receptors (Can et al., 2016). Likewise, for SERT, DAT, and NET, no inhibitory effects of ketamine or its metabolites were observed in binding or functional assays. In contrast, a set of studies do support ketamine binding to monoamine receptors and transporters. Specifically, it was found that high concentrations of ketamine (Ki = 66.8, 62.9, and 162 μM) can functionally inhibit NET, DAT, and SERT respectively (Nishimura et al., 1998). Likewise, a set of studies observed a high affinity of ketamine for the human D2 receptor expressed in Chinese Hamster Ovary cells (0.5 μM, Ki = 55 μM) and rat striatal 5-HT2 receptor (15 μM) (Kapur & Seeman, 2002; Seeman et al., 2005). Experimental conditions such as the concentration of NaCl and temperature can influence D2 receptor binding which might explain the discrepancy in these results (Can et al., 2016).
Broadly, ketamine appears to enhance monoamine signaling, which may be crucial for its antidepressant effects, much like traditional antidepressants e.g. SSRIs. Importantly, dopamine, norepinephrine, and serotonin signaling have all been associated with strengthening AMPAR signaling through phosphorylation and membrane insertion which likely contribute to the antidepressant effects of ketamine (Cai et al., 2013; Diering & Huganir, 2018; Hu et al., 2007; Sun et al., 2005). Thus, AMPARs represent a common downstream target of ketamine and its metabolites. However, the effects of ketamine on the monoamine system may also explain how ketamine elicits its negative side effects such as acute psychosis presumably through overactivation of 5-HT and D2 receptor signaling (Geyer & Vollenweider, 2008; Kokkinou et al., 2018; Schmack et al., 2021). Regarding a potential mechanism underlying these enhancements, it is unlikely that ketamine is directly binding monoamine receptors or transporters to produce its effects. Rather, ketamine is likely indirectly increasing monoamine release, perhaps through previously addressed mechanisms such as disinhibition of glutamatergic inputs into midbrain nuclei via blocking NMDARs on GABAergic neurons. Importantly, the ketamine metabolites differ considerably in their ability to modulate extracellular monoamines relative to (R) and (S)-ketamine as established by (Ago et al., 2019). (2R,6R)-HNK only produces transient increases in norepinephrine and serotonin in the PFC whereas dopamine was unchanged. Because of this, the lack of psychotomimetic and abuse potential effects seen with (2R,6R)-HNK may be due to this difference. Clinically, these phenomena are significant, as long-term use of ketamine could produce profound effects on all monoamine systems that may induce undesirable side effects compared to the more discrete effects of (2R,6R)-HNK. Certainly, more work is needed to address these concerns.
6. Conclusion
In summary, the cellular and molecular effect profile of ketamine spans across multiple neurotransmitter systems which highlights how it may rapidly and profoundly influence behavior and mood (Figure 2, Table 3). First, ketamine acts as an open-channel blocker of NMDARs (Zorumski et al., 2016). As discussed, this blockade likely contributes to changes in the release or receptor sensitivity to glutamate, GABA, BDNF, opioids, and monoamines. Namely, NMDAR blockade can lead to reductions in GABA release which disinhibits (facilitates) release of other neurotransmitters (Grunze et al., 1996). Alternatively, ketamine may block discrete postsynaptic NMDARs, such as those containing GluN2B which otherwise inhibit AMPAR signaling (Ferreira et al., 2015). This leads to synaptic strengthening through AMPAR activation which concurrently enhances the efficacy of BDNF and monoamine signaling. Additionally, ketamine and (2R,6R)-HNK may be capable of binding TrkB to facilitate BDNF signaling which drives the mechanisms necessary for structural potentiation of synapses (Casarotto et al., 2021). Enhancement in synaptic plasticity is one of the key phenomena that contributes to the antidepressant effects of ketamine (Duman et al., 2016; Riggs & Gould, 2021).
Figure 2: Model glutamatergic synapse in the prefrontal cortex (PFC) highlighting the mechanisms of ketamine action.
The actions of ketamine and its (2R,6R)-HNK metabolite have diverse actions on multiple neurotransmitter systems. Antagonism of NMDARs in GABAergic interneurons leads to a disinhibition of excitatory output due to a reduction in GABA release. Antagonism of NMDARs that prevents phosphorylation of the GluA1 subunit of AMPARs, may enhance AMPAR signaling through permitting AMPAR exchange between synaptic and extrasynaptic domains. Enhanced monoaminergic input produced by ketamine and/or (2R,6R)-HNK increases AMPAR phosphorylation at GluA1 S845 and S831 which facilitates membrane insertion, synaptic localization, and channel conductance. The BDNF receptor TrkB signals through mTOR to increase the expression of key synaptic structure proteins such as PSD95 and GluA1. Ketamine and (2R,6R)-HNK can both directly bind and activate TrkB. VTA, ventral tegmental area; DRN, dorsal raphe nucleus; LC, locus coeruleus; EPSCs, excitatory postsynaptic currents; APs, action potentials; PV, parvalbumin; PSD95, postsynaptic density-95; BDNF, brain-derived neurotrophic factor; AR, adrenergic receptor; mGluR2, metabotropic glutamate receptor 2; D1R, dopamine receptor 1; HNK, hydroxynorketamine; TrkB, tropomyosin receptor kinase B; 5-HTR, serotonin receptor; mTOR, mechanistic target of rapamycin.
Table 3: Summary of the effects of ketamine (2S,6S)-HNK, and (2R,6R)-HNK on neurotransmitter systems.
Key effects of ketamine and its (2R,6R)- and (2S,6S)-HNK metabolites discussed in this review are provided here. Notably, compared to ketamine, there is less knowledge regarding the effects of the HNKs on these neurotransmitter systems. (S) and (R) denote stereospecificity of the listed effect. PFC, prefrontal cortex; HPC, hippocampus; vlPAG, ventrolateral periaqueductal gray; DRN, dorsal raphae nucleus; NAc, nucleus accumbens; NR, nucleus reuniens; GP, globus pallidus; mGluR2/3, metabotropic glutamate receptor 2/3; BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor kinase B; 5-HT1B, serotonin receptor 1B; GAD67, glutamic acid decarboxylase 67.
| Drug | Neurotransmitter System |
||||
|---|---|---|---|---|---|
| Glutamate | GABA | Neurotrophin (BDNF) |
Opioid | Monoamine | |
| (R,S)-ketamine | NMDAR antagonist | Reduces extracellular GABA (PFC) | Increases expression (hippocampus) | (S) Full agonist at κ receptors | Increases serotonin (PFC,DRN) |
| Increases AMPAR activation | Reduces GAD67 expression | Increases release (cortical neurons) | (S) Partial agonist at μ receptors | (R) Increases norepinephrine (PFC) | |
| Increases extracellular glutamate (PFC) | Reduces parvalbumin expression | Structural enhancement of cortical and subcortical synapses | (R) Partial agonist at κ receptors | (S) Increases dopamine (PFC, NAc) | |
| Synergistic actions with mGluR2/3 antagonists | Binds and activates TrkB receptors | (R) Partial agonist at μ receptors | Increases 5-HT1B binding (HPC, NAc, GP, NR) | ||
| Increases VTA spontaneous firing | |||||
| (2R,6R)-HNK | Not a NMDAR antagonist at therapeutic concentrations | Increases release (cortical neurons) | Potential inverse agonist at μ and κ receptors | Increases serotonin (PFC) | |
| Synergistic actions with mGluR2/3 antagonists Increases AMPAR activation | Increases expression (vlPAG) | Increases norepinephrine (PFC) | |||
| Binds and activates TrkB receptors | |||||
| (2S,6S)-HNK | More potent NMDAR antagonist relative to (2R,6R)-HNK | Increases extracellular BDNF (PFC) | |||
| Lower affinity for TrkB relative to (2R,6R)-HNK | |||||
However, NMDAR blockade does not explain the mechanism of action of (2R,6R)-HNK as its affinity for and functional effects at NMDARs are beyond therapeutically relevant concentrations (Lumsden et al., 2019). Indeed, (2R,6R)-HNK does appear to differ in its pharmacodynamic effects relative to ketamine. In support, (2R,6R)-HNK produces transient increases in extracellular serotonin and norepinephrine in the PFC of mice whereas (R) and (S)-ketamine collectively produced larger, prolonged increases in extracellular serotonin, norepinephrine, and dopamine (Ago et al., 2019). Ketamine and (2R,6R)-HNK also differ in their ability to influence the opioid system. Specifically, (2R,6R)-HNK, was predicted to bind and was reported to act as an inverse agonist with IC50 values of 0.56 nM and 2.1 x 10−5 nM for the μ and κ-opioid receptor, respectively (Joseph et al., 2021). It is possible that inverse agonism of μ and κ-opioid receptors may help to restrict the potential for abuse of (2R,6R)-HNK similar to how naltrexone helps prevent relapse in opioid and alcohol use disorders, however this assertion requires further research (Anton et al., 2006; Tanum et al., 2017). On the other hand, (S) and (R)-ketamine have agonist properties at μ and κ opioid receptors, an interaction which may be necessary for their antidepressant effects (Bonaventura et al., 2021). Notably, the effects of ketamine and its stereoisomers and metabolites on NMDAR antagonism, BDNF signaling, and monoamine signaling all share a common downstream target; strengthening of AMPAR signaling (Diering & Huganir, 2018; Duman et al., 2016; Li et al., 2010; Zanos & Gould, 2018). It appears that increased strength of excitatory neurotransmission, in particular involving AMPARs, represents a common downstream target that is necessary for antidepressant efficacy among classical and rapid-acting antidepressants (Alt et al., 2006; Gould et al., 2019; Thompson et al., 2015). However, while it is important to acknowledge this mechanistic overlap, it is arguably just as important to understand the mechanisms that may render each of these compounds, and those yet to be discovered, unique. In doing so, unique mechanisms of action could potentially be mapped to efficaciousness in certain patient populations, thus more efficiently improving patient outcomes. Furthermore, as our understanding of the neurobiology of depression improves, we may find that the pharmacodynamic actions of ketamine and similar acting compounds only represent a subset of mechanisms that may be targeted for the rapid treatment of depression. Collectively, these data provide insight into the impact of ketamine and its metabolites on the treatment of depression and provide a foundation for future research into other rapid-acting antidepressants.
Acknowledgments
Supported by NIH grants T32-MH067533 from the Maryland Psychiatric Research Center (to EMH); F31-MH123066, T32-GM008181, T32-NS063391, and R25-GM055036 (to LMR); R01-MH107615 and R21-AI145211-01A1 (to TDG) and the U.S. Department of Veterans Affairs Merit Awards 101BX004062 and 101BX003631 (to TDG) and the NIDA Intramural Research Program ZIA-DA000069 (to MM).
Footnotes
CRediT Author Statement:
Evan M Hess: Writing- Original draft preparation
Lace M Riggs: Writing- Reviewing and Editing
Michael Michaelides: Writing- Reviewing and Editing
Todd D Gould: Writing- Reviewing and Editing
Declaration of Interest
E.M.H., L.M.R. and M.M. report no conflicts of interest. M.M. has received research funding from AstraZeneca, Redpin Therapeutics, and Attune Neurosciences. T.D.G. is a coauthor on patents and patent applications related to the manufacture, structure, and use of (2R,6R)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and posttraumatic stress disorder. He has assigned patent rights to the University of Maryland, Baltimore, but will share a percentage of any royalties that may be received. T.D.G. has received research funding from Allergan and Roche Pharmaceuticals and has served as a consultant for FSV7, LLC, during the preceding three years. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott JA, & Popescu GK (2020). Hydroxynorketamine Blocks N-Methyl-d-Aspartate Receptor Currents by Binding to Closed Receptors. Mol Pharmacol, 98(3), 203–210. 10.1124/mol.120.119784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson SJ, Sellman JD, Foulds JA, Frampton CM, Deering D, Dunn A, Berks J, Nixon L, & Cape G (2015). A randomized trial of combined citalopram and naltrexone for nonabstinent outpatients with co-occurring alcohol dependence and major depression. J Clin Psychopharmacol, 35(2), 143–149. 10.1097/jcp.0000000000000287 [DOI] [PubMed] [Google Scholar]
- Ago Y, Tanabe W, Higuchi M, Tsukada S, Tanaka T, Yamaguchi T, Igarashi H, Yokoyama R, Seiriki K, Kasai A, Nakazawa T, Nakagawa S, Hashimoto K, & Hashimoto H (2019). (R)-Ketamine Induces a Greater Increase in Prefrontal 5-HT Release Than (S)-Ketamine and Ketamine Metabolites via an AMPA Receptor-Independent Mechanism. Int J Neuropsychopharmacol, 22(10), 665–674. 10.1093/ijnp/pyz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, Lopez-Canul M, Torres-Berrio A, Bermudez S, Rurak GM, Simard S, Salmaso N, Gobbi G, Lacaille JC, & Sonenberg N (2021). Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature, 590(7845), 315–319. 10.1038/s41586-020-03047-0 [DOI] [PubMed] [Google Scholar]
- Ai H, Yang W, Ye M, Lu W, Yao L, & Luo JH (2011). Differential regulation of AMPA receptor GluA1 phosphorylation at serine 831 and 845 associated with activation of NMDA receptor subpopulations. Neurosci Lett, 497(2), 94–98. 10.1016/j.neulet.2011.04.038 [DOI] [PubMed] [Google Scholar]
- Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, & Kwan AC (2020). Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun, 11(1), 72. 10.1038/s41467-019-13809-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt A, Nisenbaum ES, Bleakman D, & Witkin JM (2006). A role for AMPA receptors in mood disorders. Biochem Pharmacol, 71(9), 1273–1288. 10.1016/j.bcp.2005.12.022 [DOI] [PubMed] [Google Scholar]
- Amiaz R (2019). Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism: Is It a Ketamine-Specific Effect? Am J Psychiatry, 176(3), 250–251. 10.1176/appi.ajp.2018.18111231 [DOI] [PubMed] [Google Scholar]
- Anderzhanova E, Hafner K, Genewsky AJ, Soliman A, Pöhlmann ML, Schmidt MV, Blum R, Wotjak CT, & Gassen NC (2020). The stress susceptibility factor FKBP51 controls S-ketamine-evoked release of mBDNF in the prefrontal cortex of mice. Neurobiol Stress, 13, 100239. 10.1016/j.ynstr.2020.100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, & Lodge D (1983). The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol, 79(2), 565–575. 10.1111/j.1476-5381.1983.tb11031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, & Zweben A (2006). Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama, 295(17), 2003–2017. 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, & Monteggia LM (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature, 475(7354), 91–95. 10.1038/nature10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, & Monteggia LM (2012). Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev, 64(2), 238–258. 10.1124/pr.111.005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, & Gainetdinov RR (2011). The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev, 63(1), 182–217. 10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- Belujon P, & Grace AA (2014). Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry, 76(12), 927–936. 10.1016/j.biopsych.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, & Krystal JH (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry, 47(4), 351–354. 10.1016/s0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- Bonaventura J, Lam S, Carlton M, Boehm MA, Gomez JL, Solís O, Sánchez-Soto M, Morris PJ, Fredriksson I, Thomas CJ, Sibley DR, Shaham Y, Zarate CA Jr., & Michaelides M (2021). Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry. 10.1038/s41380-021-01093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, & Keller MC (2019). No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples. Am J Psychiatry, 176(5), 376–387. 10.1176/appi.ajp.2018.18070881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, & Todd RD (2002). Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry, 51(4), 342–344. 10.1016/s0006-3223(01)01280-x [DOI] [PubMed] [Google Scholar]
- Bowman MA, Vitela M, Clarke KM, Koek W, & Daws LC (2020). Serotonin Transporter and Plasma Membrane Monoamine Transporter Are Necessary for the Antidepressant-Like Effects of Ketamine in Mice. Int J Mol Sci, 21(20). 10.3390/ijms21207581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, & Charney DS (2000). Hippocampal volume reduction in major depression. Am J Psychiatry, 157(1), 115–118. 10.1176/ajp.157.1.115 [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, & Hikosaka O (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron, 68(5), 815–834. 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Kallarackal AJ, Kvarta MD, Goluskin S, Gaylor K, Bailey AM, Lee HK, Huganir RL, & Thompson SM (2013). Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci, 16(4), 464–472. 10.1038/nn.3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, & Gould TD (2016). Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters. J Pharmacol Exp Ther, 359(1), 159–170. 10.1124/jpet.116.235838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, Martell J, Blemings A, Erritzoe D, & Nutt DJ (2021). Trial of Psilocybin versus Escitalopram for Depression. N Engl J Med, 384(15), 1402–1411. 10.1056/NEJMoa2032994 [DOI] [PubMed] [Google Scholar]
- Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, Biojone C, Cannarozzo C, Sahu MP, Kaurinkoski K, Brunello CA, Steinzeig A, Winkel F, Patil S, Vestring S, Serchov T, Diniz C, Laukkanen L, Cardon I, Antila H, Rog T, Piepponen TP, Bramham CR, Normann C, Lauri SE, Saarma M, Vattulainen I, & Castrén E (2021). Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell, 184(5), 1299–1313.e1219. 10.1016/j.cell.2021.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, & Collo G (2018). Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry, 23(4), 812–823. 10.1038/mp.2017.241 [DOI] [PubMed] [Google Scholar]
- Chaki S (2017). mGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends Pharmacol Sci, 38(6), 569–580. 10.1016/j.tips.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, Nakanishi S, & Nakazato A (2004). MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology, 46(4), 457–467. 10.1016/j.neuropharm.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Chavis P, Shinozaki H, Bockaert J, & Fagni L (1994). The metabotropic glutamate receptor types 2/3 inhibit L-type calcium channels via a pertussis toxin-sensitive G-protein in cultured cerebellar granule cells. J Neurosci, 14(11 Pt 2), 7067–7076. 10.1523/jneurosci.14-11-07067.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Nguyen TV, Pike CJ, & Russo-Neustadt AA (2007). Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal, 19(1), 114–128. 10.1016/j.cellsig.2006.05.028 [DOI] [PubMed] [Google Scholar]
- Chou D (2020). Brain-derived neurotrophic factor in the ventrolateral periaqueductal gray contributes to (2R,6R)-hydroxynorketamine-mediated actions. Neuropharmacology, 170, 108068. 10.1016/j.neuropharm.2020.108068 [DOI] [PubMed] [Google Scholar]
- Chou D, Peng HY, Lin TB, Lai CY, Hsieh MC, Wen YC, Lee AS, Wang HH, Yang PS, Chen GD, & Ho YC (2018). (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology, 139, 1–12. 10.1016/j.neuropharm.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Clements JA, Nimmo WS, & Grant IS (1982). Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci, 71(5), 539–542. 10.1002/jps.2600710516 [DOI] [PubMed] [Google Scholar]
- Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, & Caine ED (1996). Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry, 153(8), 1001–1008. 10.1176/ajp.153.8.1001 [DOI] [PubMed] [Google Scholar]
- Coull JT, Morgan H, Cambridge VC, Moore JW, Giorlando F, Adapa R, Corlett PR, & Fletcher PC (2011). Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology (Berl), 218(3), 543–556. 10.1007/s00213-011-2346-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea IA, & Naudet F (2019). US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry, 6(12), 975–977. 10.1016/s2215-0366(19)30292-5 [DOI] [PubMed] [Google Scholar]
- Currier MB, & Nemeroff CB (2014). Depression as a risk factor for cancer: from pathophysiological advances to treatment implications. Annu Rev Med, 65, 203–221. 10.1146/annurev-med-061212-171507 [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, & Drevets WC (2018). Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry, 75(2), 139–148. 10.1001/jamapsychiatry.2017.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, Lane R, Lim P, Duca AR, Hough D, Thase ME, Zajecka J, Winokur A, Divacka I, Fagiolini A, Cubala WJ, Bitter I, Blier P, Shelton RC, Molero P, Manji H, Drevets WC, & Singh JB (2019). Efficacy of Esketamine Nasal Spray Plus Oral Antidepressant Treatment for Relapse Prevention in Patients With Treatment- Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry, 76(9), 893–903. 10.1001/jamapsychiatry.2019.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, Finan PH, & Griffiths RR (2021). Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry, 78(5), 481–489. 10.1001/jamapsychiatry.2020.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Lin LA, Liu H, & Sites BD (2017). Prescription Opioid Use among Adults with Mental Health Disorders in the United States. J Am Board Fam Med, 30(4), 407–417. 10.3122/jabfm.2017.04.170112 [DOI] [PubMed] [Google Scholar]
- De Vincenti AP, Ríos AS, Paratcha G, & Ledda F (2019). Mechanisms That Modulate and Diversify BDNF Functions: Implications for Hippocampal Synaptic Plasticity. Front Cell Neurosci, 13, 135. 10.3389/fncel.2019.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, & Soderling TR (2007). Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci, 8(2), 101–113. 10.1038/nrn2055 [DOI] [PubMed] [Google Scholar]
- Deyama S, & Duman RS (2020). Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol Biochem Behav, 188, 172837. 10.1016/j.pbb.2019.172837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar AK, & Barton DA (2016). Depression and the Link with Cardiovascular Disease. Front Psychiatry, 7, 33. 10.3389/fpsyt.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, & Huganir RL (2018). The AMPA Receptor Code of Synaptic Plasticity. Neuron, 100(2), 314–329. 10.1016/j.neuron.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF (2010). Taming the ketamine tiger. 1965. Anesthesiology, 113(3), 678–684. 10.1097/ALN.0b013e3181ed09a2 [DOI] [PubMed] [Google Scholar]
- Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF, & Traynelis SF (2007). Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol, 581(Pt 1), 107–128. 10.1113/jphysiol.2006.124958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR Jr., Todd RD, Reich T, Vannier M, & Raichle ME (1997). Subgenual prefrontal cortex abnormalities in mood disorders. Nature, 386(6627), 824–827. 10.1038/386824a0 [DOI] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Cajina M, Müller HK, Elfving B, Sanchez C, & Wegener G (2017). S-Ketamine Mediates Its Acute and Sustained Antidepressant-Like Activity through a 5-HT(1B) Receptor Dependent Mechanism in a Genetic Rat Model of Depression. Front Pharmacol, 8, 978. 10.3389/fphar.2017.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, & Krystal JH (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med, 22(3), 238–249. 10.1038/nm.4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, & Nestler EJ (1997). A molecular and cellular theory of depression. Arch Gen Psychiatry, 54(7), 597–606. 10.1001/archpsyc.1997.01830190015002 [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, & Duman RS (2013). mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry, 1(1), 15. 10.1186/2049-9256-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzubay JA, & Jahr CE (1999). The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J Neurosci, 19(13), 5265–5274. 10.1523/jneurosci.19-13-05265.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, & Weinberger DR (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112(2), 257–269. 10.1016/s0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- Entsuah AR, Huang H, & Thase ME (2001). Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry, 62(11), 869–877. 10.4088/jcp.v62n1106 [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, & Malinow R (2003). PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci, 6(2), 136–143. 10.1038/nn997 [DOI] [PubMed] [Google Scholar]
- Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, & Lucki I (2016). Antidepressant-like Effects of Buprenorphine are Mediated by Kappa Opioid Receptors. Neuropsychopharmacology, 41(9), 2344–2351. 10.1038/npp.2016.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JM (2001). SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J Clin Psychiatry, 3(1), 22–27. 10.4088/pcc.v03n0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JS, Papouin T, Ladépêche L, Yao A, Langlais VC, Bouchet D, Dulong J, Mothet JP, Sacchi S, Pollegioni L, Paoletti P, Oliet SHR, & Groc L (2017). Co-agonists differentially tune GluN2B-NMDA receptor trafficking at hippocampal synapses. Elife, 6. 10.7554/eLife.25492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JS, Schmidt J, Rio P, Águas R, Rooyakkers A, Li KW, Smit AB, Craig AM, & Carvalho AL (2015). GluN2B-Containing NMDA Receptors Regulate AMPA Receptor Traffic through Anchoring of the Synaptic Proteasome. J Neurosci, 35(22), 8462–8479. 10.1523/jneurosci.3567-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck AD, & Ngai SH (1982). Opiate receptor mediation of ketamine analgesia. Anesthesiology, 56(4), 291–297. 10.1097/00000542-198204000-00011 [DOI] [PubMed] [Google Scholar]
- Fratta W, Casu M, Balestrieri A, Loviselli A, Biggio G, & Gessa GL (1980). Failure of ketamine to interact with opiate receptors. Eur J Pharmacol, 61(4), 389–391. 10.1016/0014-2999(80)90079-5 [DOI] [PubMed] [Google Scholar]
- Fred SM, Laukkanen L, Brunello CA, Vesa L, Göös H, Cardon I, Moliner R, Maritzen T, Varjosalo M, Casarotto PC, & Castrén E (2019). Pharmacologically diverse antidepressants facilitate TRKB receptor activation by disrupting its interaction with the endocytic adaptor complex AP-2. J Biol Chem, 294(48), 18150–18161. 10.1074/jbc.RA119.008837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Schüle C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Möller HJ, & Meisenzahl EM (2007). Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry, 64(4), 410–416. 10.1001/archpsyc.64.4.410 [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Fogaca MV, Liu RJ, Duman C, Kato T, Li XY, & Duman RS (2019). Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A, 116(1), 297–302. 10.1073/pnas.1814709116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, & Chaki S (2016). The Antidepressant Effects of an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacology, 41(4), 1046–1056. 10.1038/npp.2015.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldon C, Papola D, Ostuzzi G, & Barbui C (2019). Esketamine for treatment resistant depression: a trick of smoke and mirrors? Epidemiol Psychiatr Sci, 29, e79. 10.1017/s2045796019000751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneva, W. H. O. (2020). Global Health Estimates 2019: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000-2019. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys
- George MS (2018). Is There Really Nothing New Under the Sun? Is Low-Dose Ketamine a Fast-Acting Antidepressant Simply Because It Is An Opioid? Am J Psychiatry, 175(12), 1157–1158. 10.1176/appi.ajp.2018.18070800 [DOI] [PubMed] [Google Scholar]
- Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, Taylor SR, Duman CH, Delpire E, Picciotto M, Wohleb ES, & Duman RS (2020). GABA interneurons are the cellular trigger for ketamine's rapid antidepressant actions. J Clin Invest, 130(3), 1336–1349. 10.1172/jci130808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, & Vollenweider FX (2008). Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci, 29(9), 445–453. 10.1016/j.tips.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Götz R, Raulf F, & Schartl M (1992). Brain-derived neurotrophic factor is more highly conserved in structure and function than nerve growth factor during vertebrate evolution. J Neurochem, 59(2), 432–442. 10.1111/j.1471-4159.1992.tb09389.x [DOI] [PubMed] [Google Scholar]
- Gould TD, Zanos P, & Zarate CA Jr. (2017). Ketamine Mechanism of Action: Separating the Wheat from the Chaff. Neuropsychopharmacology, 42(1), 368–369. 10.1038/npp.2016.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Zarate CA Jr., & Thompson SM (2019). Molecular Pharmacology and Neurobiology of Rapid-Acting Antidepressants. Annu Rev Pharmacol Toxicol, 59, 213–236. 10.1146/annurev-pharmtox-010617-052811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Simes M, Berman R, Koenigsberg SH, & Kessler RC (2021). The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics, 39(6), 653–665. 10.1007/s40273-021-01019-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, & Greene RW (1996). NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci, 16(6), 2034–2043. 10.1523/jneurosci.16-06-02034.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, Swanson GT, Swanger SA, Greger IH, Nakagawa T, McBain CJ, Jayaraman V, Low CM, Dell'Acqua ML, Diamond JS, Camp CR, Perszyk RE, Yuan H, & Traynelis SF (2021). Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol Rev, 73(4), 298–487. 10.1124/pharmrev.120.000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Williams NR, Bentzley BS, & Schatzberg AF (2019). Rigorous Trial Design Is Essential to Understand the Role of Opioid Receptors in Ketamine's Antidepressant Effect. JAMA Psychiatry, 76(6), 657–658. 10.1001/jamapsychiatry.2019.0766 [DOI] [PubMed] [Google Scholar]
- Heifets BD, Williams NR, Blasey C, Sudheimer K, Rodriguez CI, & Schatzberg AF (2019a). Interpreting Ketamine's Opioid Receptor Dependent Effect: Response to Sanacora. Am J Psychiatry, 176(3), 249–250. 10.1176/appi.ajp.2018.18091061r [DOI] [PubMed] [Google Scholar]
- Heifets BD, Williams NR, Blasey C, Sudheimer K, Rodriguez CI, & Schatzberg AF (2019b). Target Population, Dose, and Timing Considerations for Understanding Naltrexone's Subjective Effect: Response to Amiaz. Am J Psychiatry, 176(3), 251–252. 10.1176/appi.ajp.2018.18111231r [DOI] [PubMed] [Google Scholar]
- Henley JM, & Wilkinson KA (2016). Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci, 17(6), 337–350. 10.1038/nrn.2016.37 [DOI] [PubMed] [Google Scholar]
- Henriksson MM, Aro HM, Marttunen MJ, Heikkinen ME, Isometsä ET, Kuoppasalmi KI, & Lönnqvist JK (1993). Mental disorders and comorbidity in suicide. Am J Psychiatry, 150(6), 935–940. 10.1176/ajp.150.6.935 [DOI] [PubMed] [Google Scholar]
- Highland JN, Morris PJ, Zanos P, Lovett J, Ghosh S, Wang AQ, Zarate CA Jr., Thomas CJ, Moaddel R, & Gould TD (2019). Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of (2R,6R)-hydroxynorketamine. J Psychopharmacol, 33(1), 12–24. 10.1177/0269881118812095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland JN, Zanos P, Riggs LM, Georgiou P, Clark SM, Morris PJ, Moaddel R, Thomas CJ, Zarate CA Jr., Pereira EFR, & Gould TD (2021). Hydroxynorketamines: Pharmacology and Potential Therapeutic Applications. Pharmacol Rev, 73(2), 763–791. 10.1124/pharmrev.120.000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM, & Porter JH (2014). Ketamine, but not MK-801, produces antidepressant-like effects in rats responding on a differential-reinforcement-of-low-rate operant schedule. Behav Pharmacol, 25(1), 80–91. 10.1097/fbp.0000000000000014 [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH, & Negus SS (2014). Comparison of antidepressant-like and abuse-related effects of phencyclidine in rats. Drug Dev Res, 75(8), 479–488. 10.1002/ddr.21228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Okawa H, Appadu BL, Grandy DK, Devi LA, & Lambert DG (1999). Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology, 90(1), 174–182. 10.1097/00000542-199901000-00023 [DOI] [PubMed] [Google Scholar]
- Höflich A, Kraus C, Pfeiffer RM, Seiger R, Rujescu D, Zarate CA Jr., Kasper S, Winkler D, & Lanzenberger R (2021). Translating the immediate effects of S-Ketamine using hippocampal subfield analysis in healthy subjects-results of a randomized controlled trial. Transl Psychiatry, 11(1), 200. 10.1038/s41398-021-01318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, Nabulsi N, Matuskey D, Angarita GA, Pietrzak RH, Duman RS, Sanacora G, Krystal JH, Carson RE, & Esterlis I (2019). Lower synaptic density is associated with depression severity and network alterations. Nat Commun, 10(1), 1529. 10.1038/s41467-019-09562-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RI, de Groot M, & Golden SH (2014). Diabetes and depression. Curr Diab Rep, 14(6), 491. 10.1007/s11892-014-0491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, & Moghaddam B (2007). NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci, 27(43), 11496–11500. 10.1523/jneurosci.2213-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, & Jonas P (2014). Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science, 345(6196), 1255263. 10.1126/science.1255263 [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, & Malinow R (2007). Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell, 131(1), 160–173. 10.1016/j.cell.2007.09.017 [DOI] [PubMed] [Google Scholar]
- Hustveit O, Maurset A, & Oye I (1995). Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol, 77(6), 355–359. 10.1111/j.1600-0773.1995.tb01041.x [DOI] [PubMed] [Google Scholar]
- Itoi K, & Sugimoto N (2010). The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol, 22(5), 355–361. 10.1111/j.1365-2826.2010.01988.x [DOI] [PubMed] [Google Scholar]
- Jentsch JD, & Roth RH (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology, 20(3), 201–225. 10.1016/s0893-133x(98)00060-8 [DOI] [PubMed] [Google Scholar]
- Joseph TT, Bu W, Lin W, Zoubak L, Yeliseev A, Liu R, Eckenhoff RG, & Brannigan G (2021). Ketamine Metabolite (2R,6R)-Hydroxynorketamine Interacts with μ and κ Opioid Receptors. ACS Chem Neurosci, 12(9), 1487–1497. 10.1021/acschemneuro.0c00741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, & Baudry M (2009). Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci, 29(27), 8688–8697. 10.1523/jneurosci.6078-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, & Seeman P (2002). NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry, 7(8), 837–844. 10.1038/sj.mp.4001093 [DOI] [PubMed] [Google Scholar]
- Kessler RC (2004). The epidemiology of dual diagnosis. Biol Psychiatry, 56(10), 730–737. 10.1016/j.biopsych.2004.06.034 [DOI] [PubMed] [Google Scholar]
- Klein ME, Chandra J, Sheriff S, & Malinow R (2020). Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci U S A, 117(5), 2656–2662. 10.1073/pnas.1916570117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinou M, Ashok AH, & Howes OD (2018). The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry, 23(1), 59–69. 10.1038/mp.2017.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski SE, & Johnson JW (2009). Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer's drug memantine. J Neurosci, 29(9), 2774–2779. 10.1523/jneurosci.3703-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimmel SR, Zanos P, Georgiou P, Colloca L, & Gould TD (2020). Classical conditioning of antidepressant placebo effects in mice. Psychopharmacology (Berl), 237(1), 93–102. 10.1007/s00213-019-05347-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., & Charney DS (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry, 51(3), 199–214. 10.1001/archpsyc.1994.03950030035004 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Yoon G, & Petrakis IL (2019). Rigorous Trial Design Is Essential to Understand the Role of Opioid Receptors in Ketamine's Antidepressant Effect-Reply. JAMA Psychiatry, 76(6), 658–659. 10.1001/jamapsychiatry.2019.0763 [DOI] [PubMed] [Google Scholar]
- Kuner T, & Schoepfer R (1996). Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci, 16(11), 3549–3558. 10.1523/jneurosci.16-11-03549.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, & Zarate C Jr. (2012). Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry, 72(11), e27–28. 10.1016/j.biopsych.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, & Murrough JW (2014). A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry, 76(12), 970–976. 10.1016/j.biopsych.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasič E, Lisjak M, Horvat A, Božić M, Šakanović A, Anderluh G, Verkhratsky A, Vardjan N, Jorgačevski J, Stenovec M, & Zorec R (2019). Astrocyte Specific Remodeling of Plasmalemmal Cholesterol Composition by Ketamine Indicates a New Mechanism of Antidepressant Action. Sci Rep, 9(1), 10957. 10.1038/s41598-019-47459-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Bang E, Lee B, Dwyer JM, & Duman RS (2016). Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology, 111, 242–252. 10.1016/j.neuropharm.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, & Duman RS (2014). BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol, 18(1). 10.1093/ijnp/pyu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, & Duman RS (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329(5994), 959–964. 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QS, Wajs E, Ochs-Ross R, Singh J, & Drevets WC (2020). Genome-wide association study and polygenic risk score analysis of esketamine treatment response. Sci Rep, 10(1), 12649. 10.1038/s41598-020-69291-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA (2006). Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov, 5(2), 160–170. 10.1038/nrd1958 [DOI] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, & Aghajanian GK (2012). Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry, 71(11), 996–1005. 10.1016/j.biopsych.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lin D, Wu B, & Zhou W (2016). Ketamine abuse potential and use disorder. Brain Res Bull, 126(Pt 1), 68–73. 10.1016/j.brainresbull.2016.05.016 [DOI] [PubMed] [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, Kessler RC, & Kossowsky J (2017). Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis. JAMA Psychiatry, 74(10), 1011–1020. 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gil X, Jiménez-Sánchez L, Campa L, Castro E, Frago C, & Adell A (2019). Role of Serotonin and Noradrenaline in the Rapid Antidepressant Action of Ketamine. ACS Chem Neurosci, 10(7), 3318–3326. 10.1021/acschemneuro.9b00288 [DOI] [PubMed] [Google Scholar]
- Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, Lovett J, Kim S, Wang FH, Schmidt S, Jenne CE, Yuan P, Morris PJ, Thomas CJ, Zarate CA Jr., Moaddel R, Traynelis SF, Pereira EFR, Thompson SM, Albuquerque EX, & Gould TD (2019). Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci U S A, 116(11), 5160–5169. 10.1073/pnas.1816071116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, & Pennefather P (1987). Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol, 58(2), 251–266. 10.1152/jn.1987.58.2.251 [DOI] [PubMed] [Google Scholar]
- MacDonald JF, & Nowak LM (1990). Mechanisms of blockade of excitatory amino acid receptor channels. Trends Pharmacol Sci, 11(4), 167–172. 10.1016/0165-6147(90)90070-o [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G, & Manji HK (2008). Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry, 63(4), 349–352. 10.1016/j.biopsych.2007.05.028 [DOI] [PubMed] [Google Scholar]
- Marton T, Barnes DE, Wallace A, & Woolley JD (2019). Concurrent Use of Buprenorphine, Methadone, or Naltrexone Does Not Inhibit Ketamine's Antidepressant Activity. Biol Psychiatry, 85(12), e75–e76. 10.1016/j.biopsych.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Matsuda K, Fletcher M, Kamiya Y, & Yuzaki M (2003). Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. J Neurosci, 23(31), 10064–10073. 10.1523/jneurosci.23-31-10064.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, & Pfrieger FW (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science, 294(5545), 1354–1357. 10.1126/science.294.5545.1354 [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Prickaerts J, & Steinbusch HW (2008). The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer's disease. Prog Brain Res, 172, 233–264. 10.1016/s0079-6123(08)00912-6 [DOI] [PubMed] [Google Scholar]
- Minichiello L (2009). TrkB signalling pathways in LTP and learning. Nat Rev Neurosci, 10(12), 850–860. 10.1038/nrn2738 [DOI] [PubMed] [Google Scholar]
- Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, & Wainer IW (2013). Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol, 698(1-3), 228–234. 10.1016/j.ejphar.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Venkata SL, Tanga MJ, Bupp JE, Green CE, Iyer L, Furimsky A, Goldberg ME, Torjman MC, & Wainer IW (2010). A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta, 82(5), 1892–1904. 10.1016/j.talanta.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, & Liston C (2019). Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science, 364(6436). 10.1126/science.aat8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, & Seeburg PH (1992). Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science, 256(5060), 1217–1221. 10.1126/science.256.5060.1217 [DOI] [PubMed] [Google Scholar]
- Moore JW, Cambridge VC, Morgan H, Giorlando F, Adapa R, & Fletcher PC (2013). Time, action and psychosis: using subjective time to investigate the effects of ketamine on sense of agency. Neuropsychologia, 51(2), 377–384. 10.1016/j.neuropsychologia.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA Jr., & Thomas CJ (2017). Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites. Org Lett, 19(17), 4572–4575. 10.1021/acs.orglett.7b02177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, & Kalivas PW (2011). Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci, 5, 94. 10.3389/fnsys.2011.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Paine TA, Rittiner JE, Béguin C, Carroll FI, Roth BL, Cohen BM, & Carlezon WA Jr. (2010). Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl), 210(2), 263–274. 10.1007/s00213-010-1834-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, & Duman RS (1995). Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci, 15(11), 7539–7547. 10.1523/jneurosci.15-11-07539.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, & Duman RS (1996). Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci, 16(7), 2365–2372. 10.1523/jneurosci.16-07-02365.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, & Nichols CD (2008). Serotonin Receptors. Chemical Reviews, 108(5), 1614–1641. 10.1021/cr078224o [DOI] [PubMed] [Google Scholar]
- Niesters M, Khalili-Mahani N, Martini C, Aarts L, van Gerven J, van Buchem MA, Dahan A, & Rombouts S (2012). Effect of subanesthetic ketamine on intrinsic functional brain connectivity: a placebo-controlled functional magnetic resonance imaging study in healthy male volunteers. Anesthesiology, 117(4), 868–877. 10.1097/ALN.0b013e31826a0db3 [DOI] [PubMed] [Google Scholar]
- Niesters M, Martini C, & Dahan A (2014). Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol, 77(2), 357–367. 10.1111/bcp.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, & Tohyama M (1998). Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells. Anesthesiology, 88(3), 768–774. 10.1097/00000542-199803000-00029 [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, & Kavalali ET (2013). Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci, 33(16), 6990–7002. 10.1523/jneurosci.4998-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, & Soderling TR (2006). Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem, 281(2), 752–758. 10.1074/jbc.M509677200 [DOI] [PubMed] [Google Scholar]
- Omranifard V, Shirzadi E, Samandari S, Afshar H, & Maracy MR (2014). Memantine add on to citalopram in elderly patients with depression: A double-blind placebo-controlled study. J Res Med Sci, 19(6), 525–530. [PMC free article] [PubMed] [Google Scholar]
- Ouakinin SRS, Barreira DP, & Gois CJ (2018). Depression and Obesity: Integrating the Role of Stress, Neuroendocrine Dysfunction and Inflammatory Pathways. Front Endocrinol (Lausanne), 9, 431. 10.3389/fendo.2018.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco Dda F, Romero TR, & Duarte ID (2014). Central antinociception induced by ketamine is mediated by endogenous opioids and μ- and δ-opioid receptors. Brain Res, 1562, 69–75. 10.1016/j.brainres.2014.03.026 [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, & Zhou Q (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci, 14(6), 383–400. 10.1038/nrn3504 [DOI] [PubMed] [Google Scholar]
- Paoletti P, & Neyton J (2007). NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol, 7(1), 39–47. 10.1016/j.coph.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Parsons CG, Panchenko VA, Pinchenko VO, Tsyndrenko AY, & Krishtal OA (1996). Comparative patch-clamp studies with freshly dissociated rat hippocampal and striatal neurons on the NMDA receptor antagonistic effects of amantadine and memantine. Eur J Neurosci, 8(3), 446–454. 10.1111/j.1460-9568.1996.tb01228.x [DOI] [PubMed] [Google Scholar]
- Peciña M, Karp JF, Mathew S, Todtenkopf MS, Ehrich EW, & Zubieta JK (2019). Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol Psychiatry, 24(4), 576–587. 10.1038/s41380-018-0117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Otaño I, Larsen RS, & Wesseling JF (2016). Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat Rev Neurosci, 17(10), 623–635. 10.1038/nrn.2016.92 [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, & Heinemann SF (2001). Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci, 21(4), 1228–1237. 10.1523/jneurosci.21-04-01228.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, Dackis CA, & O'Brien CP (2010). A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. Am J Psychiatry, 167(6), 668–675. 10.1176/appi.ajp.2009.08060852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, & Emrich HM (1986). Psychotomimesis mediated by kappa opiate receptors. Science, 233(4765), 774–776. 10.1126/science.3016896 [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, & Gardier AM (2017). Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology, 112(Pt A), 198–209. 10.1016/j.neuropharm.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Picard N, Takesian AE, Fagiolini M, & Hensch TK (2019). NMDA 2A receptors in parvalbumin cells mediate sex-specific rapid ketamine response on cortical activity. Mol Psychiatry, 24(6), 828–838. 10.1038/s41380-018-0341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothula S, Kato T, Liu RJ, Wu M, Gerhard D, Shinohara R, Sliby AN, Chowdhury GMI, Behar KL, Sanacora G, Banerjee P, & Duman RS (2020). Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol Psychiatry. 10.1038/s41380-020-0796-3 [DOI] [PubMed] [Google Scholar]
- Pothula S, Liu RJ, Wu M, Sliby AN, Picciotto MR, Banerjee P, & Duman RS (2021). Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons. Neuropsychopharmacology, 46(4), 799–808. 10.1038/s41386-020-00882-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi L, Pollak Dorocic I, Wang X, Carlén M, & Meletis K (2014). Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS One, 9(1), e83879. 10.1371/journal.pone.0083879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziejewski C, Robinson RC, DiStefano PS, & Taylor JW (1992). Dimeric structure and conformational stability of brain-derived neurotrophic factor and neurotrophin-3. Biochemistry, 31(18), 4431–4436. 10.1021/bi00133a007 [DOI] [PubMed] [Google Scholar]
- Rantamäki T, Vesa L, Antila H, Di Lieto A, Tammela P, Schmitt A, Lesch KP, Rios M, & Castrén E (2011). Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS One, 6(6), e20567. 10.1371/journal.pone.0020567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs LM, Aracava Y, Zanos P, Fischell J, Albuquerque EX, Pereira EFR, Thompson SM, & Gould TD (2020). (2R,6R)-hydroxynorketamine rapidly potentiates hippocampal glutamatergic transmission through a synapse-specific presynaptic mechanism. Neuropsychopharmacology, 45(2), 426–436. 10.1038/s41386-019-0443-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs LM, & Gould TD (2021). Ketamine and the Future of Rapid-Acting Antidepressants. Annu Rev Clin Psychol, 17, 207–231. 10.1146/annurev-clinpsy-072120-014126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, & Fava M (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry, 163(11), 1905–1917. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Sanacora G (2019). Caution Against Overinterpreting Opiate Receptor Stimulation as Mediating Antidepressant Effects of Ketamine. Am J Psychiatry, 176(3), 249. 10.1176/appi.ajp.2018.18091061 [DOI] [PubMed] [Google Scholar]
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, Burke MA, Zajecka JM, Barra L, Su HL, Posener JA, Bui KH, Quirk MC, Piser TM, Mathew SJ, & Pathak S (2017). Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study. Neuropsychopharmacology, 42(4), 844–853. 10.1038/npp.2016.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, & Quirk MC (2014). Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry, 19(9), 978–985. 10.1038/mp.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler M (1990). Monoamine oxidase inhibitors in depression: history and mythology. J Psychopharmacol, 4(3), 136–139. 10.1177/026988119000400307 [DOI] [PubMed] [Google Scholar]
- Schmack K, Bosc M, Ott T, Sturgill JF, & Kepecs A (2021). Striatal dopamine mediates hallucination-like perception in mice. Science, 372(6537). 10.1126/science.abf4740 [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, & Tallerico T (2005). Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics. Mol Psychiatry, 10(9), 877–883. 10.1038/sj.mp.4001682 [DOI] [PubMed] [Google Scholar]
- Shaffer CL, Dutra JK, Tseng WC, Weber ML, Bogart LJ, Hales K, Pang J, Volfson D, Am Ende CW, Green ME, & Buhl DL (2019). Pharmacological evaluation of clinically relevant concentrations of (2R,6R)-hydroxynorketamine. Neuropharmacology, 153, 73–81. 10.1016/j.neuropharm.2019.04.019 [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, & Mizuno N (1997). Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci, 17(19), 7503–7522. 10.1523/jneurosci.17-19-07503.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NS, Rutkowska E, Plazinska A, Khadeer M, Moaddel R, Jozwiak K, Bernier M, & Wainer IW (2016). Ketamine Metabolites Enantioselectively Decrease Intracellular D-Serine Concentrations in PC-12 Cells. PLoS One, 11(4), e0149499. 10.1371/journal.pone.0149499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Pekoe GM, Martin LL, & Coalgate B (1980). The interaction of ketamine with the opiate receptor. Life Sci, 26(10), 789–795. 10.1016/0024-3205(80)90285-4 [DOI] [PubMed] [Google Scholar]
- Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, & Rothschild AJ (2013). Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry, 74(10), 966–973. 10.4088/JCP.12m08252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethling J, Le L, & Meaney DF (2012). NMDA receptor mediated phosphorylation of GluR1 subunits contributes to the appearance of calcium-permeable AMPA receptors after mechanical stretch injury. Neurobiol Dis, 46(3), 646–654. 10.1016/j.nbd.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies M, James GM, Berroterán-Infante N, Ibeschitz H, Kranz GS, Unterholzner J, Godbersen M, Gryglewski G, Hienert M, Jungwirth J, Pichler V, Reiter B, Silberbauer L, Winkler D, Mitterhauser M, Stimpfl T, Hacker M, Kasper S, & Lanzenberger R (2018). Assessment of Ketamine Binding of the Serotonin Transporter in Humans with Positron Emission Tomography. Int J Neuropsychopharmacol, 21(2), 145–153. 10.1093/ijnp/pyx085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg AD (1993). Structure, function, and regulation of adrenergic receptors. Protein Sci, 2(8), 1198–1209. 10.1002/pro.5560020802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, & Wolf ME (2005). Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci, 25(32), 7342–7351. 10.1523/jneurosci.4603-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, & Monteggia LM (2017). Effects of a ketamine metabolite on synaptic NMDAR function. Nature, 546(7659), E1–e3. 10.1038/nature22084 [DOI] [PubMed] [Google Scholar]
- Tanum L, Solli KK, Latif ZE, Benth J, Opheim A, Sharma-Haase K, Krajci P, & Kunøe N (2017). Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA Psychiatry, 74(12), 1197–1205. 10.1001/jamapsychiatry.2017.3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenore PL (2008). Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis, 27(3), 49–65. 10.1080/10550880802122646 [DOI] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, VanMeter S, Harriett AE, & Wang Y (2005). Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry, 66(8), 974–981. 10.4088/jcp.v66n0803 [DOI] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, & Cai X (2015). An excitatory synapse hypothesis of depression. Trends Neurosci, 38(5), 279–294. 10.1016/j.tins.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiger M, Veldman ER, Ekman CJ, Halldin C, Svenningsson P, & Lundberg J (2020). A randomized placebo-controlled PET study of ketamine’s effect on serotonin(1B) receptor binding in patients with SSRI-resistant depression. Transl Psychiatry, 10(1), 159. 10.1038/s41398-020-0844-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, & Dingledine R (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev, 62(3), 405–496. 10.1124/pr.109.002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, & Fava M (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry, 163(1), 28–40. 10.1176/appi.ajp.163.1.28 [DOI] [PubMed] [Google Scholar]
- Trullas R, & Skolnick P (1990). Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol, 185(1), 1–10. 10.1016/0014-2999(90)90204-j [DOI] [PubMed] [Google Scholar]
- Turner EH (2019). Esketamine for treatment-resistant depression: seven concerns about efficacy and FDA approval. Lancet Psychiatry, 6(12), 977–979. 10.1016/s2215-0366(19)30394-3 [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, & Duman RS (1997). 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci, 17(8), 2785–2795. 10.1523/jneurosci.17-08-02785.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoose AM, Clements JM, & Winder DG (2006). Novel blockade of protein kinase A-mediated phosphorylation of AMPA receptors. J Neurosci, 26(4), 1138–1145. 10.1523/jneurosci.3572-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, & Shaham Y (2020). Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci, 21(11), 625–643. 10.1038/s41583-020-0378-z [DOI] [PubMed] [Google Scholar]
- Wang N, Zhang GF, Liu XY, Sun HL, Wang XM, Qiu LL, Yang C, & Yang JJ (2014). Downregulation of neuregulin 1-ErbB4 signaling in parvalbumin interneurons in the rat brain may contribute to the antidepressant properties of ketamine. J Mol Neurosci, 54(2), 211–218. 10.1007/s12031-014-0277-8 [DOI] [PubMed] [Google Scholar]
- Widman AJ, & McMahon LL (2018). Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A, 115(13), E3007–e3016. 10.1073/pnas.1718883115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, & Schatzberg AF (2018). Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am J Psychiatry, 175(12), 1205–1215. 10.1176/appi.ajp.2018.18020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, & Rorick-Kehn LM (2016). The Rapidly Acting Antidepressant Ketamine and the mGlu2/3 Receptor Antagonist LY341495 Rapidly Engage Dopaminergic Mood Circuits. J Pharmacol Exp Ther, 358(1), 71–82. 10.1124/jpet.116.233627 [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Yokoyama C, Mizuma H, Kurai S, Finnema SJ, Halldin C, Doi H, & Onoe H (2014). A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: a PET study with macaques. Transl Psychiatry, 4(1), e342. 10.1038/tp.2013.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, & Hashimoto K (2020). Correction: R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry, 10(1), 295. 10.1038/s41398-020-00983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Petrakis IL, & Krystal JH (2019). Association of Combined Naltrexone and Ketamine With Depressive Symptoms in a Case series of Patients With Depression and Alcohol Use Disorder. JAMA Psychiatry, 76(3), 337–338. 10.1001/jamapsychiatry.2018.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, & Gould TD (2018). Intracellular Signaling Pathways Involved in (S)- and (R)-Ketamine Antidepressant Actions. Biol Psychiatry, 83(1), 2–4. 10.1016/j.biopsych.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, Morris PJ, Stewart BW, Thomas CJ, Thompson SM, Moaddel R, & Gould TD (2019). (R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br J Pharmacol, 176(14), 2573–2592. 10.1111/bph.14683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, Morris PJ, Thomas CJ, Moaddel R, Zarate CA Jr., & Gould TD (2019). (2R,6R)-hydroxynorketamine exerts mGlu(2) receptor-dependent antidepressant actions. Proc Natl Acad Sci U S A, 116(13), 6441–6450. 10.1073/pnas.1819540116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., & Gould TD (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature, 533(7604), 481–486. 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., & Gould TD (2018). Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev, 70(3), 621–660. 10.1124/pr.117.015198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, & Wainer IW (2012). Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry, 72(4), 331–338. 10.1016/j.biopsych.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, & Luckenbaugh DA (2013). A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry, 74(4), 257–264. 10.1016/j.biopsych.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, & Manji HK (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry, 63(8), 856–864. 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, & Charney DS (2006). A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry, 163(1), 153–155. 10.1176/appi.ajp.163.1.153 [DOI] [PubMed] [Google Scholar]
- Zhang F, Hillhouse TM, Anderson PM, Koppenhaver PO, Kegen TN, Manicka SG, Lane JT, Pottanat E, Van Fossen M, Rice R, & Porter JH (2021). Opioid receptor system contributes to the acute and sustained antidepressant-like effects, but not the hyperactivity motor effects of ketamine in mice. Pharmacol Biochem Behav, 208, 173228. 10.1016/j.pbb.2021.173228 [DOI] [PubMed] [Google Scholar]
- Zhang K, & Hashimoto K (2019). Lack of Opioid System in the Antidepressant Actions of Ketamine. Biol Psychiatry, 85(6), e25–e27. 10.1016/j.biopsych.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Zhang K, Xu T, Yuan Z, Wei Z, Yamaki VN, Huang M, Huganir RL, & Cai X (2016). Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal, 9(458), ra123. 10.1126/scisignal.aai7884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Yamaki VN, Wei Z, Zheng Y, & Cai X (2017). Differential regulation of GluA1 expression by ketamine and memantine. Behav Brain Res, 316, 152–159. 10.1016/j.bbr.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ye F, Zhang T, Lv S, Zhou L, Du D, Lin H, Guo F, Luo C, & Zhu S (2021). Structural basis of ketamine action on human NMDA receptors. Nature, 596(7871), 301–305. 10.1038/s41586-021-03769-9 [DOI] [PubMed] [Google Scholar]
- Zhao X, Venkata SL, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA Jr., Mager DE, & Wainer IW (2012). Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol, 74(2), 304–314. 10.1111/j.1365-2125.2012.04198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Kyzer JL, Worob A, & Wenthur CJ (2021). Family of Structurally Related Bioconjugates Yields Antibodies with Differential Selectivity against Ketamine and 6-Hydroxynorketamine. ACS Chem Neurosci, 12(21), 4113–4122. 10.1021/acschemneuro.1c00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhang G, Li X, Liu X, Wang N, Qiu L, Liu W, Zuo Z, & Yang J (2015). Loss of phenotype of parvalbumin interneurons in rat prefrontal cortex is involved in antidepressant- and propsychotic-like behaviors following acute and repeated ketamine administration. Mol Neurobiol, 51(2), 808–819. 10.1007/s12035-014-8798-2 [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y, & Mennerick S (2016). Ketamine: NMDA Receptors and Beyond. J Neurosci, 36(44), 11158–11164. 10.1523/jneurosci.1547-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]