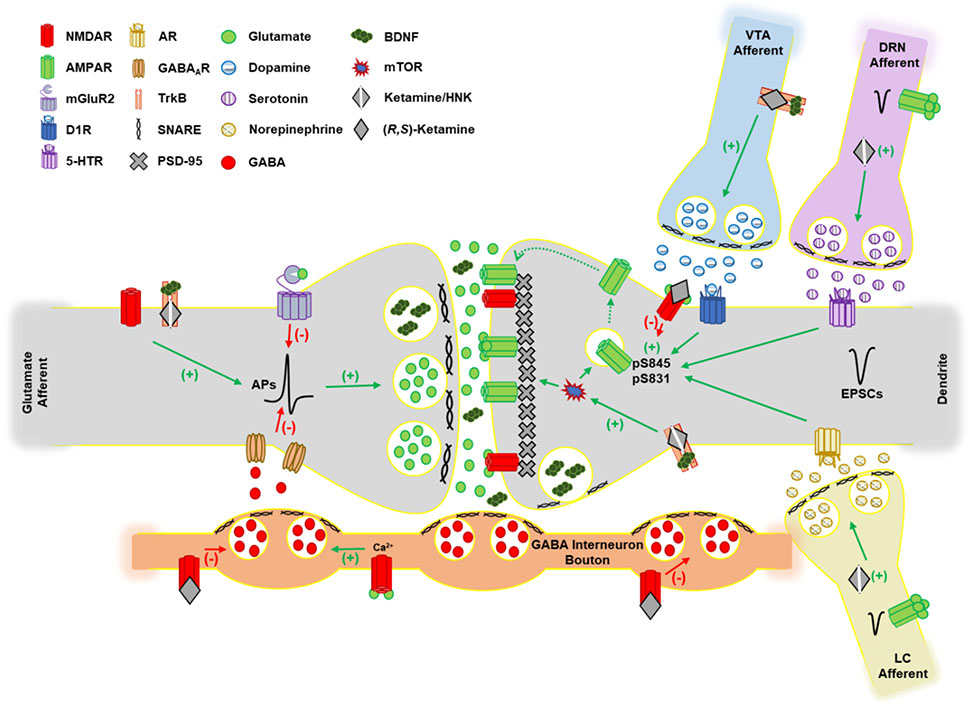
Figure 2: Model glutamatergic synapse in the prefrontal cortex (PFC) highlighting the mechanisms of ketamine action.
The actions of ketamine and its (2R,6R)-HNK metabolite have diverse actions on multiple neurotransmitter systems. Antagonism of NMDARs in GABAergic interneurons leads to a disinhibition of excitatory output due to a reduction in GABA release. Antagonism of NMDARs that prevents phosphorylation of the GluA1 subunit of AMPARs, may enhance AMPAR signaling through permitting AMPAR exchange between synaptic and extrasynaptic domains. Enhanced monoaminergic input produced by ketamine and/or (2R,6R)-HNK increases AMPAR phosphorylation at GluA1 S845 and S831 which facilitates membrane insertion, synaptic localization, and channel conductance. The BDNF receptor TrkB signals through mTOR to increase the expression of key synaptic structure proteins such as PSD95 and GluA1. Ketamine and (2R,6R)-HNK can both directly bind and activate TrkB. VTA, ventral tegmental area; DRN, dorsal raphe nucleus; LC, locus coeruleus; EPSCs, excitatory postsynaptic currents; APs, action potentials; PV, parvalbumin; PSD95, postsynaptic density-95; BDNF, brain-derived neurotrophic factor; AR, adrenergic receptor; mGluR2, metabotropic glutamate receptor 2; D1R, dopamine receptor 1; HNK, hydroxynorketamine; TrkB, tropomyosin receptor kinase B; 5-HTR, serotonin receptor; mTOR, mechanistic target of rapamycin.