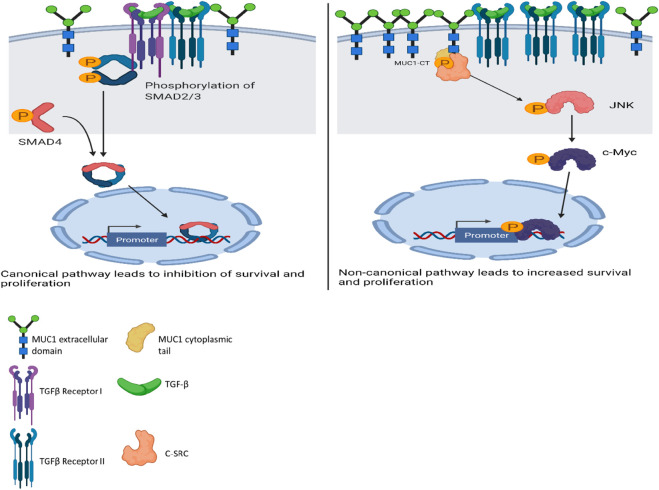
FIGURE 6.
Schematic diagram of the proposed mechanism of TGF-β signaling and functions in high versus low MUC1 PDA. Left panel shows activation of SMAD-dependent canonical pathway in low-MUC1 PDA cells. TGF-β ligands bind to the membranous TGF-β receptor (TGF-βRII) homodimers with high affinity. TGF-βRII binding allows dimerization with TGF-β type I receptor (TGF-βRI) homodimers, activation of the TGF-βRI kinase domain and signal transduction via phosphorylation of the C-terminus of receptor-regulated SMADs (R-SMAD), SMAD2 and SMAD3. The SMAD2/3 dimer then forms a heterotrimeric complex with SMAD4 which translocates in the nucleus (Massagué and Wotton, 2000; Ross and Hill, 2008). This leads to growth inhibition, cell cycle arrest and apoptosis of PDA cells, thus TGF-β acts as a tumor suppressor. Right panel shows activation of SMAD-independent non-canonical pathway in high-MUC1 PDA cells. In this pathway, binding of TGF-β mainly to TGF-β-RII most likely increases phosphorylation of c-SRC which in turn phosphorylates MAPK, followed by JNK and c-Myc (Bunda et al., 2014). This phosphorylation cascade activates the MAPK/JNK pathway and stabilizes c-Myc which translocates into the nucleus to increase transcription of oncogenic proteins and leads to increased growth, invasion and EMT of PDA cells (Fey et al., 2016). MUC1-CT also aids in the process by its oncogenic signaling. Thus, in high-MUC1 PDA cells TGF-β acts as a pro-tumorigenic cytokine. The schematic was created with BioRender.com.